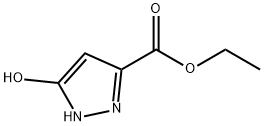
Ethyl 5-Oxo-4,5-dihydro-1H-pyrazole-3-carboxylate synthesis
- Product Name:Ethyl 5-Oxo-4,5-dihydro-1H-pyrazole-3-carboxylate
- CAS Number:85230-37-1
- Molecular formula:C6H8N2O3
- Molecular Weight:156.14
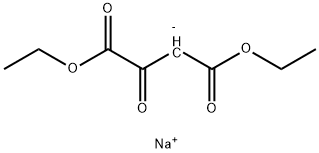
40876-98-0

85230-37-1
The general procedure for the synthesis of ethyl 5-keto-4,5-dihydro-1H-pyrazole-3-carboxylate from the sodium salt of diethyl oxaloacetate was as follows: acetic acid (150 mL) was slowly added dropwise to a solution of 1,4-diethoxy-1,4-dioxobut-2-en-2-ol (30.0 g, 0.143 mol) in toluene (150 mL). The reaction mixture was stirred at room temperature for 30 minutes before 85% hydrazine hydrochloride (17 g, 0.29 mol) was added. Stirring was continued at room temperature for 30 minutes, after which the reaction system was heated to 100 °C and kept overnight. Upon completion of the reaction, the mixture was concentrated under reduced pressure and extracted with ethyl acetate (500 mL). The organic phase was washed sequentially with saturated aqueous sodium bicarbonate (200 mL) and saturated aqueous sodium chloride (200 mL), dried over anhydrous sodium sulfate and filtered, and concentrated under reduced pressure to give a yellow solid product. The yield was 17 g (0.11 mol) with 77% yield. The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 12.75 (br s, 1H), 5.91 (br s, 1H), 4.24 (q, J = 7 Hz, 2H), 1.27 (t, J = 7 Hz, 3H).
Yield: 92%
Reaction Conditions:
Stage #1:diethyl oxalacetate sodium salt in benzene for 0.333333 h;
Stage #2: with acetic acid for 0.5 h;
Stage #3:hydrazine hydrochloride at 100; for 24.5 h;Heating / reflux;
Steps:
1 Example 1; 5-HYDROXY-LH-PYRAZOLE-3-CARBOXYLIC acid ethyl ester
Diethyloxalacetate, sodium salt (14. 53 g, 69. 15 mmol) was dissolved in 100 mL of benzene and stirred for 20 min. To the solution was added 100 ML of acetic acid and the reaction mixture was stirred for a further 30 min. Hydrazine monohydrochloride (9. 47 g, 138 mmol) was added and the reaction mixture was stirred for an additional 30 min. The reaction was brought to reflux at 100 C for 24 h. The reaction was then removed from heat and cooled to room temperature and extracted with ethyl acetate and washed with 10% hydrochloric acid, saturated sodium bicarbonate solution, water and then brine. The solvent was removed IN VACUO TO yield an oily solid which was then triturated with a 2 : 1 mixture of diethyl ether : hexanes to yield 3 (10. 00 g, 92%) as an off-white solid : LRMS (electrospray) ; m/z [M+H1+ = 157.
References:
F. HOFFMANN-LA ROCHE AG WO2004/74257, 2004, A1 Location in patent:Page 26; 37; 38

64-17-5
758 suppliers
$10.00/50g

3702-98-5
59 suppliers
$17.67/250mgs:

85230-37-1
96 suppliers
$58.00/1g


