
Ethyl 5-hydroxyindole-2-carboxylate synthesis
- Product Name:Ethyl 5-hydroxyindole-2-carboxylate
- CAS Number:24985-85-1
- Molecular formula:C11H11NO3
- Molecular Weight:205.21
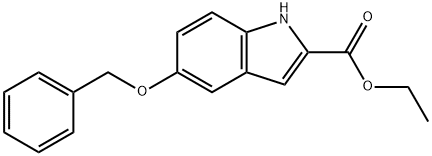
37033-95-7

24985-85-1
General procedure for the synthesis of ethyl 5-hydroxyindole-2-carboxylate from ethyl 5-benzyloxyindole-2-carboxylate: ethyl 5-benzyloxyindole-2-carboxylate (10 g, 34 mmol) was dissolved in ethanol (250 mL), 10% Pd/C catalyst was added, and the hydrogenation reaction was carried out for 20 h at atmospheric pressure (using a double-walled balloon). Upon completion of the reaction, the reaction mixture was diluted with ethyl acetate and filtered through diatomaceous earth to remove the catalyst. The filtrate was concentrated in vacuum to give ethyl 5-hydroxyindole-2-carboxylate (7 g, 99% yield) in brown solid form. The product was characterized by 1H NMR (400 MHz, CDCl3): δ 8.86 (broad peak, 1H), 7.29 (d, J = 8.80 Hz, 1H), 7.11 (s, 1H), 7.06 (s, 1H), 6.94 (d, J = 8.70 Hz, 1H), 4.83 (broad peak, 1H), 4.42 (q, J = 7.09 Hz, 2H ), 1.41 (t, J = 7.08Hz, 3H).

37033-95-7
135 suppliers
$35.09/Price $/250mgs:

24985-85-1
132 suppliers
$19.00/100mg
Yield:24985-85-1 99%
Reaction Conditions:
with hydrogen;10% palladium on activated carbon in ethanol; for 20 h;
Steps:
1.c Scheme 1; EXAMPLE 1; Preparation of 5-(2-Chloro-5-hydroxybenzyloxy)-1-[(2-thiophen-3-yl)ethyl]-1H-indole-2-carboxylic Acid; c) 5-Hydroxyindole-2-carboxylic Acid Ethyl Ester
A solution of 5-benzyloxyindole-2-carboxylic acid ethyl ester (10 g, 34 mmol) in ethanol (250 mL) was treated with 10% Pd/C and hydrogenated under atmospheric pressure (double-walled balloon) for 20 h. The reaction mixture was diluted with EtOAc and filtered through Celite. Evaporation of the filtrate in vacuo gave the title compound as a tan solid (7 g, 99%). 1H NMR (400 MHz, CDCl3) δ 8.86 (br s, 1H), 7.29 (d, J=8.80 Hz, 1H), 7.11 (s, 1H), 7.06 (s, 1H), 6.94 (d, J=8.70 Hz, 1H), 4.83 (br s, 1H), 4.42 (q, J=7.09 Hz, 2H), 1.41 (t, J=7.08 Hz, 3H).
References:
US6670388,2003,B1 Location in patent:Page column 6
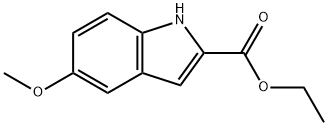
4792-58-9
157 suppliers
$29.00/1g

24985-85-1
132 suppliers
$19.00/100mg

21598-06-1
146 suppliers
$29.00/250mg

64-17-5
760 suppliers
$10.00/50g

24985-85-1
132 suppliers
$19.00/100mg
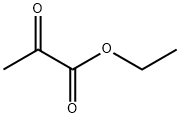
617-35-6
560 suppliers
$5.00/10g

123-30-8
628 suppliers
$10.00/5g

24985-85-1
132 suppliers
$19.00/100mg

21598-06-1
146 suppliers
$29.00/250mg

24985-85-1
132 suppliers
$19.00/100mg