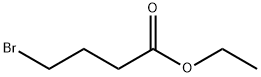
Ethyl 4-bromobutyrate synthesis
- Product Name:Ethyl 4-bromobutyrate
- CAS Number:2969-81-5
- Molecular formula:C6H11BrO2
- Molecular Weight:195.05

2623-87-2

2969-81-5
Part A: Synthesis of ethyl 4-bromobutyrate To a solution of 4-bromobutyric acid (3 g, 18 mmol) in ethanol (30 mL) was added 5 mL of a dioxane solution of 4N HCl. The reaction mixture was stirred at room temperature for 16 hours. Upon completion of the reaction, the volatile components were removed under vacuum. The concentrated residue was dissolved in 150 mL of dichloromethane. The organic phase was washed sequentially with saturated aqueous NaHCO3 (1 x 150 mL) and brine (1 x 150 mL), dried over anhydrous MgSO4, filtered, and the filtrate was concentrated under reduced pressure. The concentrated residue was dried under vacuum to give 3 g (85.5% yield) of ethyl 4-bromobutyrate as a yellow oil.1H NMR (300 MHz, CDCl3): δ1.22 (t, 3H, J = 7.15 Hz), 2.13 (m, 2H, J = 6.80 Hz), 2.45 (t, 2H, J = 7.15 Hz), 3.43 (t , 2H, J = 6.44Hz), 4.10 (q, 2H, J = 7.25Hz).
Yield:2969-81-5 93.47%
Reaction Conditions:
Stage #1: ?-butyrolactonewith hydrogen bromide at 20 - 40;
Stage #2: ethanol at 40;
Steps:
1 Experimental group 1:
Add 200g γ-butyrolactone to a 500mL three-necked flask,The control temperature is 20±5,Continue after slowly feeding the dry hydrogen bromide gas of 226g (1.2 times of amount, mol ratio),The temperature was raised to 40±5, and the stirring reaction should be continued for 1±0.5 hours.Add 140mL (1.06 times the amount, molar ratio) of absolute ethanol,Continue to control the temperature to be 40±5°C, and stir until the reaction is completed.After the reaction was completed, 200 mL of deionized water was added to wash, and the mixture was fully stirred.The layers were left to stand, the lower organic phase was taken out, and the pH was adjusted to 7.0 with saturated NaHCO3 solution.Let stand for stratification, separate the lower organic phase, add 200 mL of deionized water to wash,Stir well, stand for stratification, separate the lower organic phase,After drying with a desiccant (molecular sieve or anhydrous sodium sulfate), the product 4-bromobutyric acid ethyl ester is obtained. By gas chromatography analysis, no by-products. The yield was 93.47%, and the purity was 98.27%.
References:
CN114736119,2022,A Location in patent:Paragraph 0045-0048

64-17-5
760 suppliers
$10.00/50g
927-58-2
194 suppliers
$6.00/250mg

2969-81-5
453 suppliers
$5.00/1g

999-10-0
59 suppliers
inquiry

2969-81-5
453 suppliers
$5.00/1g


