
Ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate synthesis
- Product Name:Ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate
- CAS Number:74892-82-3
- Molecular formula:C9H17NO2
- Molecular Weight:171.24
![2-Piperidinecarboxylic acid, 4-methyl-1-[(1S)-1-phenylethyl]-, ethyl ester, (2R,4R)-](/CAS/20210305/GIF/198641-56-4.gif)
198641-56-4

74892-82-3
General procedure for the synthesis of ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate from the compound (CAS: 198641-56-4): (1'S,2R,4R)-6 (2.5 g, 9 mmol) was dissolved in ethanol (120 mL) and 10% Pd(OH)2/C (0.27 g) was added. The hydrogenation reaction was carried out at room temperature and at atmospheric pressure. The reaction progress was monitored by TLC (unfolding agent: chloroform/methanol, 9:1). After 4 hours of reaction, the catalyst was removed by filtration through a diatomaceous earth pad. The filtrate was concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography (silica gel to sample mass ratio 1:20) to afford pure ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate (0.7 g, 45% yield) using dichloromethane/methanol (98:2) as eluent. The product was characterized by 1H NMR (CDCl3): δ 0.96 (d, 3H, J = 6.50 Hz, CH3-4); 1.15 (m, 1H, H-5a); 1.32 (t, 3H, J = 7.2 Hz, CH3CH2); 1.42-1.54 (m, 1H, H-3); 1.5-1.69 (m, 2H, H-4, H-5b) ; 2.00-2.11 (m, 2H, H-3b and NH); 2.87 (m, 2H, H-6); 3.65 (m, 1H, H-2); 4.21 (q, 2H, J = 7.2 Hz, CH2CH3). Specific optical rotation [α]20D = -22 (c 5, ethanol) (enantiomeric excess value e.e. = +24).
![2-Piperidinecarboxylic acid, 4-methyl-1-[(1S)-1-phenylethyl]-, ethyl ester, (2R,4R)-](/CAS/20210305/GIF/198641-56-4.gif)
198641-56-4
0 suppliers
inquiry

74892-82-3
222 suppliers
$80.00/500mg
Yield:74892-82-3 345 g
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen;iron(II) oxalate;acetic acid in ethanol at 30; under 3750.38 Torr; for 4 h;Autoclave;Reagent/catalyst;
Steps:
1.2 two,(2R, 4R) -4-methylpiperidine-2-carboxylate Preparation
The crude product (618 g) obtained in the previous step and ethanol (2 mL) were charged into a 5 L autoclave,Additional acetic acid (45 g), palladium on carbon catalyst (10% palladium loading, 15 g), 3 g iron oxalate,Access to H2, at 30 , 0.5MPa reaction 4h,filter,Catalyst recovery.The filtrate was concentrated under reduced pressure and added with ethyl acetate (1 L)Washed sequentially with saturated sodium carbonate solution (250 mL × 2), saturated brine (250 mL × 2)Dried over anhydrous sodium sulfate, filtered,The filtrate was concentrated under reduced pressure,Have a pale yellow liquid product.The resulting crude product was purified by distillation,Collected 89 ~ 90 / 10mmHg fractions,345 g of ethyl (2R, 4R) -4-methyl-2-piperidinecarboxylate was obtained in a yield of 91%.Optical rotation: (c = 5, EtOH)The total process yield above: 82.45%.Liquid chromatography determination:The (2R, 4R) -4-methyl-2-piperidinecarboxylic acid ethyl ester content was 99.3%.
References:
CN107043347,2017,A Location in patent:Paragraph 0029; 0030; 0031; 0032-0034; 0039-0044; 0046; 0048
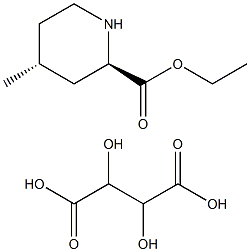
131278-84-7
11 suppliers
inquiry

74892-82-3
222 suppliers
$80.00/500mg
![(2R)-1,2,3,6-Tetrahydro-4-Methyl-1-[(1R)-1-phenylethyl]-2-pyridinecarboxylic Acid Ethyl Ester](/CAS/20150408/GIF/139334-62-6.gif)
139334-62-6
6 suppliers
inquiry

74892-82-3
222 suppliers
$80.00/500mg
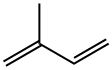
78-79-5
262 suppliers
$14.56/25ML

74892-82-3
222 suppliers
$80.00/500mg

139334-63-7
1 suppliers
inquiry

74892-82-3
222 suppliers
$80.00/500mg