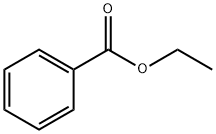
Ethyl 2-bromobenzoate synthesis
- Product Name:Ethyl 2-bromobenzoate
- CAS Number:6091-64-1
- Molecular formula:C9H9BrO2
- Molecular Weight:229.07

64-17-5
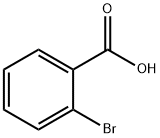
88-65-3

6091-64-1
O-bromobenzoic acid (5 g, 24.87 mmol) was dissolved in anhydrous ethanol (100 mL). Concentrated sulfuric acid (5 mL) was slowly added as a catalyst, a reflux condensing unit was installed, and the system was heated to reflux temperature in an oil bath with continuous stirring for 24 hours. After the reaction was completed, the reaction system was cooled to room temperature. Most of the ethanol solvent was removed by concentration under reduced pressure by rotary evaporator. The residue was transferred to a partition funnel, washed with deionized water to remove acidic impurities, and subsequently extracted with ethyl acetate (3 x 50 mL). The organic phases were combined and dried with anhydrous magnesium sulfate for 30 min. The desiccant was removed by filtration and the filtrate was concentrated by rotary evaporation to obtain the crude product. Finally, the crude product was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate=10:1) to afford ethyl 2-bromobenzoate (4.9 g, 86% yield) as a colorless oily liquid.
Yield: 47%
Reaction Conditions:
in tetrahydrofuran for 120 h;Reflux;
Steps:
Ethyl 2-Bromobenzoate; Typical Procedure for Ethylation of Weaker Acids
Sulfonimidate 1 (0.859 g, 3.00 mmol) and 2-bromobenzoic acid (0.601 g, 3.00 mmol) were refluxed in THF (25 mL) for 5 d. TLC analysis (hexane-EtOAc, 6:1) showed that most of 1 had been converted into the sulfonamide 2. The mixture was concentrated by rotary evaporation and pentane (5 mL) was added. The pentane solution was removed by pipet and treated with NaH to precipitate any unreacted acid and residual 2. This solution was pipet filtered, concentrated in vacuo and purified by Kugelrohr distillation; yield: 0.32 g (47%). IR and 1H NMR spectra were identical to published spectra.
References:
Maricich, Tom J.;Allan, Matthew J.;Kislin, Brett S.;Chen, Andrea I-T.;Meng, Fan-Chun;Bradford, Christine;Kuan, Nai-Chia;Wood, Jeremy;Aisagbonhi, Omonigho;Poste, Alethea;Wride, Dustin;Kim, Sylvia;Santos, Therese;Fimbres, Michael;Choi, Dianne;Elia, Haydi;Kaladjian, Joseph;Abou-Zahr, Ali;Mejia, Arturo [Synthesis,2013,vol. 45,# 24,art. no. SS-2013-M0543-OP,p. 3361 - 3368]
93-89-0
528 suppliers
$13.00/100g

6091-64-1
177 suppliers
$10.00/1g

64-17-5
758 suppliers
$10.00/50g
7154-66-7
171 suppliers
$10.00/5g

6091-64-1
177 suppliers
$10.00/1g