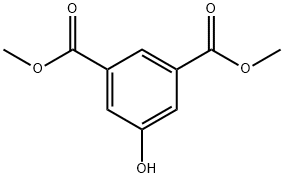
Dimethyl 5-hydroxyisophthalate synthesis
- Product Name:Dimethyl 5-hydroxyisophthalate
- CAS Number:13036-02-7
- Molecular formula:C10H10O5
- Molecular Weight:210.18

67-56-1
618-83-7

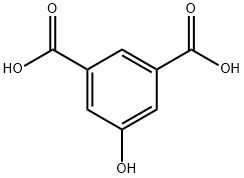
13036-02-7
To a 1 L round-bottomed flask equipped with a magnetic stirrer was added 187.8 g (1 mol) of 5-hydroxyisophthalic acid (~97% purity, available from Aldrich) and 500 mL (12.3 mol) of methanol. After slowly adding 28 mL of concentrated sulfuric acid, the reaction mixture was heated to reflux temperature and stirred under reflux conditions for 4 hours. Upon completion of the reaction, the hot reaction solution was carefully poured into 500mL of ice water. Subsequently, the white solid product was collected by filtration and washed several times with deionized water until the washings were neutral. The product was dried under vacuum at 60 °C overnight to give 206.8 g of dimethyl 5-hydroxyisophthalate. The product characterization data were as follows: melting point 164-166 °C; GC purity >99%; yield 98.2%; 1H NMR (CDCl3 + DMSO-d6, TMS as internal standard, ppm): 3.68 (s, 6H, OCH3), 7.45 (s, 2H, Ar-H), 7.89 (s, 1H, Ar-H), 9.35 (broad peak, 1H, OH ); 13C NMR (CDCl3 + DMSO-d6, TMS as internal standard, ppm): 51.92 (OCH3), 120.50 (C4, C6), 121.10 (C2), 131.15 (C1, C3), 157.36 (C5), 165.85 (COOR); 1H-13C NMR correlation spectrum (CDCl3 + DMSO-d6, TMS as internal standard, ppm): 51.92 (quadruple peaks, OCH3, 1JCH = 147 Hz), 120.50 (double peaks, C4, C6, 1JCH = 164.4 Hz), 121.10 (double peaks, C2, 1JCH = 168.3 Hz), 131.15 (single peaks, C1, C3), 157.36 (single peaks , C5), 165.85 (single peak, COOR); GC-MS (acetone as solvent): m/z 210 (M+).

618-83-7
310 suppliers
$9.00/10g

13036-02-7
180 suppliers
$6.00/5g
Yield:13036-02-7 100%
Steps:
Examples; The following compounds were prepared by the method described below: Table 1: Compounds related to the general Formula (I) Empirical melting point E/ mol-1cm-1 Compound No. Formula/°C No.max. (solvent DMSO) (Ia) C30H28N2O5 174 288 34500 (Ib) C30H28N205 157 302 26600 (Ic) C40H48N2O5 151-153 283 17600 (Id) C3oH26Cl2N2O5 218 307 25500 (Ie) C30H26Cl2N2O5 195 308 27600 (If) C30H24Cl4N2O5 198 314 27400 (Ig) C38H44N205 192-193 304 24700 Said compounds can be obtained, for example, by a multistep synthesis starting from 5- hydroxyisophthalic acid (1). (Reference e. g. G. J.Bodwell, J. N.Bridson, M.K.Cyranski, J. W.J.Kennedy, T. M.Krygowski, M. R.Mannion and D. O.Miller, J. Org.Chem, 68 (6), 2089-2098 (2003)) which is first converted under standard conditions and nearly quantitative yields into its dimethylester (2).
References:
WO2005/118562,2005,A1 Location in patent:Page/Page column 8
![Dimethyl 5-[[(Trifluoromethyl)sulfonyl]oxy]isophthalate](/CAS/20180703/GIF/168619-21-4.gif)
168619-21-4
7 suppliers
inquiry

13036-02-7
180 suppliers
$6.00/5g

618-83-7
310 suppliers
$9.00/10g

18107-18-1
210 suppliers
$33.00/5g

13036-02-7
180 suppliers
$6.00/5g
