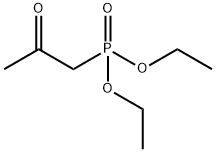
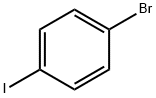
DIETHYL 4-BROMOBENZYL PHOSPHONATE synthesis
- Product Name:DIETHYL 4-BROMOBENZYL PHOSPHONATE
- CAS Number:38186-51-5
- Molecular formula:C11H16BrO3P
- Molecular Weight:307.12

589-15-1
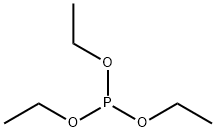
122-52-1

38186-51-5
General procedure for the synthesis of diethyl 4-bromobenzylphosphonate (16) from p-bromobenzyl bromide and triethyl phosphite: 1-bromo-4-(bromomethyl)benzene (5.0 g, 20 mmol) and triethyl phosphite (51 mL, 300 mmol) were added to a round-bottomed flask, and the mixture was reacted by refluxing the reaction for 19 hours at 90 °C. Upon completion of the reaction, the excess triethyl phosphite was removed by distillation under reduced pressure. The crude product was purified by fast column chromatography (eluent ratio 1:1 hexane/ethyl acetate) to afford the target compound 16 in 98% yield. The product was a colorless liquid; 1H NMR (400 MHz, CDCl3) δ 7.30 (d, 2H, J = 7.5 Hz), 7.05 (d, 2H, J = 7.6 Hz), 3.99-3.88 (m, 4H), 2.99 (s, 1H), 2.94 (s, 1H), 1.12 (t, 6H, J = 6.9 Hz); 13C NMR ( 100 MHz, CDCl3) δ 131.7, 131.6, 131.5, 121.0, 62.3, 34.0, 32.0, 16.5; HRMS calculated value (C11H16BrO3P, [M + H]+) 307.0097, measured value 307.0093.
Yield: 90%
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide at 120; for 24 h;Inert atmosphere;
Steps:
3 Example 3 Triethyl phosphite and 4-bromobenzyl alcohol were used to prepare diethyl 4-bromobenzylphosphonate
To a 20 mL tubular reactor was added 4-bromobenzyl alcohol (93.0 mg, 0.50 mmol)(3.8 mg, 0.01 mmol, 2 mol%), protected by vacuum nitrogen and then heated to 120 ° C under solvent-free conditions 24h After the TLC monitoring reaction was complete, the product was purified by column chromatography and the yield was 90%.
References:
Wenzhou University;Xu Qing;Ma Xiantao;Su Chenliang;Han Libiao CN106543221, 2017, A Location in patent:Paragraph 0030; 0031; 0032; 0033

19350-68-6
11 suppliers
inquiry
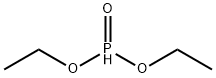
762-04-9
347 suppliers
$10.00/10 g

38186-51-5
111 suppliers
$8.00/1g