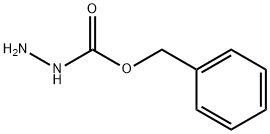
Carbobenzoxyhydrazide synthesis
- Product Name:Carbobenzoxyhydrazide
- CAS Number:5331-43-1
- Molecular formula:C8H10N2O2
- Molecular Weight:166.18
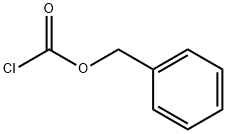
501-53-1

5331-43-1
1. General procedure for the synthesis of benzyl hydrazinecarboxylate: In a three-necked reaction flask equipped with temperature control, hydrazine hydrate (2.0 mol), potassium carbonate (2.0 mol), and 50 mL of tetrahydrofuran are added sequentially. After cooling the reaction system to -20°C, benzyl chloroformate (2.0 mol) was slowly added. After completion of the reaction, stirring was continued for 2 h. The potassium carbonate was subsequently removed by filtration and washed with water. The organic phase was concentrated to give the crude product of benzyl hydrazinecarboxylate (95.0% yield, 96.0% purity as determined by high performance gas chromatography (GC)). 2. Purification of the crude product of benzyl hydrazinecarboxylate: In another three-necked reaction flask, add the crude product of benzyl hydrazinecarboxylate (40.0 g, purity 96.0%, determined by high performance gas chromatography (GC)) and 400 mL of dichloromethane. 80 mL of 30% hydrochloric acid was added dropwise to the reaction system and solid precipitation was observed. 150 mL of water was added to dissolve some of the solids. The organic layer was separated and retained. Add 80 mL of ammonia dropwise to the aqueous layer and adjust the pH to alkaline. The aqueous layer was extracted three times with 250 mL of dichloromethane. The organic phases were combined and concentrated to give purified benzyl hydrazinecarboxylate (36.0 g, 99.5% purity, 90.0% yield as determined by high performance gas chromatography (GC)).

501-53-1
416 suppliers
$10.00/1g

5331-43-1
378 suppliers
$6.00/10g
Yield:5331-43-1 97%
Reaction Conditions:
with potassium carbonate;hydrazine hydrate in dichloromethane at 20; for 2 h;Reagent/catalyst;Solvent;Temperature;
Steps:
1-3 A method for preparing benzyl carbazate includes the following steps:
The first step: add dichloromethane solvent to the reaction kettle, then add hydrazine hydrate and stir to dissolve, then add the catalyst, continue to stir for at least 5 minutes; the weight ratio of the hydrazine hydrate and the solvent is 1:6 in sequence; the catalyst is potassium carbonate , Sodium carbonate, lithium carbonate, triethylamine, potassium hydroxide or sodium hydroxide;Step 2: At 20±2, slowly drip benzyl chloroformate into the reaction kettle, the dripping time is controlled at 10min, and the reaction is kept for 2h; hydrazine hydrate, catalyst and benzyl chloroformate are in order according to the molar ratio 16:1:4;The third step: heat the obtained reaction liquid through a liquid separation device for heat preservation and water separation. After water separation, hydrazine hydrate is recovered and used, and the organic phase obtained after water separation is used for later use;The fourth step: add 1mol/L hydrochloric acid to the organic phase obtained after water separation for acidification refining;Step 5: Add sodium carbonate aqueous solution to the acidified and refined solution for neutralization; the neutralized solution is cooled and crystallized by centrifugation, and the temperature for cooling and crystallization is 0°C; the filtrate is separated to remove water, and the organic phase is recovered and used for crystallization. The solids are collected for use;Step 6: Recrystallize the collected crystalline solid with water to obtain benzyl carbazate.
References:
CN113336676,2021,A Location in patent:Paragraph 0018-0026

22129-07-3
11 suppliers
inquiry

5331-43-1
378 suppliers
$6.00/10g
3459-92-5
137 suppliers
$17.46/1G

5331-43-1
378 suppliers
$6.00/10g

202980-89-0
0 suppliers
inquiry

5331-43-1
378 suppliers
$6.00/10g

