
BOC-VAL-NH2 synthesis
- Product Name:BOC-VAL-NH2
- CAS Number:35150-08-4
- Molecular formula:C10H20N2O3
- Molecular Weight:216.28

13734-41-3

35150-08-4
General procedure for the synthesis of tert-butyl (S)-(1-amino-3-methyl-1-oxo-2-butyl)carbamate from Boc-L-valine: to a colorless solution of 150 mg (0.50 mmol) of Boc-L-valine in 10 mL of THF was added 67 μL (0.70 mmol, 1.4 eq.) of ethyl chloroformate at 0 °C and 209 μL ( 1.5 mmol, 3.0 eq.) triethylamine. After stirring at 0 °C for 30 min, 0.75 mL of 1.0 M aqueous ammonium chloride (0.75 mmol, 1.5 equiv) was added to the colorless suspension. The mixture was continued to be stirred at 0 °C for 30 min, followed by the addition of 5 mL of water. The colorless clear solution was extracted with 30 mL of ethyl acetate and the aqueous layer was extracted with another 20 mL of ethyl acetate. The organic layers were combined, washed with 5 mL of brine and dried with anhydrous magnesium sulfate. The crude product was purified by silica gel column chromatography (eluent: ethyl acetate) to give 129 mg (86% yield) of tert-butyl (S)-(1-amino-3-methyl-1-oxo-2-butyl)carbamate. The product was a colorless solid, melting point: 149-152 °C; [α]30D = -2.4 (c 1.00, methanol); 1H NMR (400 MHz, CDCl3): δ 0.94 (d, J = 6.8 Hz, 3H, CH3CH), 0.99 (d, J = 6.8 Hz, 3H, CH3CH), 1.45 (s, 9H, (CH3)3C ), 2.16 (ddd, J = 6.7,6.8,6.8 Hz, 1H, CH(CH3)2), 3.96 (dd, J = 6.7,7.8 Hz, 1H, CHCO), 5.03,5.42,5.89 (br, br, br, 1H, 1H, 1H, NH, NH2); 13C NMR (100 MHz, CDCl3) : δ17.8,19.3,28.3,30.8,59.5,79.9,156.0,174.4; IR (KBr, vmax/cm-1): 3386 (CONH), 3345 (CONH), 3205 (CONH), 1680 (CON), 1641 (CON); HRMS (ESI-TOF): Calculated value for C10H20N2O3Na (M + Na)+: 239.1366, measured value: 239.1340. Enantiomeric ratio was determined by HPLC (Chiralcel OD column: hexane/2-propanol = 95/5): retention time 9.4 min.

13734-41-3
564 suppliers
$8.00/1g

35150-08-4
86 suppliers
$11.00/250mg
Yield: 98%
Reaction Conditions:
Stage #1:t-Boc-L-valine with chloroformic acid ethyl ester;triethylamine in tetrahydrofuran at 0; for 0.5 h;
Stage #2: with ammonium chloride in tetrahydrofuran;water at 0; for 0.5 h;
Steps:
4.3.11 4.3. Typical procedure for the primary amidation of Cbz-l-Phe-OH 3a with NH4Cl
General procedure: To a colorless solution of 150mg (0.50mmol) of Cbz-L-Phe-OH 3a in 10mL of THF were added 67μL (0.70mmol, 1.4equiv) of ClCO2Et and 209μL (1.5mmol, 3.0equiv) of Et3N at 0°C.
After stirring for 30min at 0°C, 0.75 ml of a 1.0M aqueous solution of NH4Cl (0.75mmol, 1.5equiv) were added at 0°C to the colorless suspension.
The mixture was stirred for 30min at 0°C and 5mL of H2O was added to the resulted mixture.
The colorless clear solution was extracted with 30mL of EtOAc and the aqueous layer was extracted with 20mL of EtOAc.
The organic layers were combined, washed with 5mL of brine, and dried over anhydrous MgSO4.
The crude product was chromatographed on silica gel with EtOAc to afford 129mg (86% yield) of Cbz-L-Phe-NH2 4a. 4.3.11
Boc-l-Val-NH2 4f
106?mg (98%); >99% ee; coloress solid; mp: 149-152?°C; [α]30D?=?-2.4 (c 1.00, MeOH); 1H NMR (400?MHz, CDCl3): δ 0.94 (d, J?=?6.8?Hz, 3H, CH3CH), 0.99 (d, J?=?6.8?Hz, 3H, CH3CH), 1.45 (s, 9H, (CH3)3C), 2.16 (ddd, J?=?6.7, 6.8, 6.8?Hz, 1H, CH(CH3)2), 3.96 (dd, J?=?6.7, 7.8?Hz, 1H, CHCO), 5.03, 5.42, 5.89 (br, br, br, 1H, 1H, 1H, NH, NH2); 13C NMR (100?MHz, CDCl3): δ 17.8, 19.3, 28.3, 30.8, 59.5, 79.9, 156.0, 174.4; IR (KBr, vmax/cm-1): 3386 (CONH), 3345 (CONH), 3205 (CONH), 1680 (CON), 1641 (CON); HRMS (ESI-TOF): Calcd for C10H20N2O3Na (M+Na)+: 239.1366, found: 239.1340; The enantiomeric ratio was determined by HPLC (Chiralcel OD: hexane/2-propanol?=?95/5): Tr 9.4?min.
References:
Ezawa, Tetsuya;Kawashima, Yuya;Noguchi, Takuya;Jung, Seunghee;Imai, Nobuyuki [Tetrahedron Asymmetry,2017,vol. 28,# 12,p. 1690 - 1699]
![Carbamic acid, N-[(1S)-1-acetyl-2-methylpropyl]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/128211-52-9.gif)
128211-52-9
2 suppliers
inquiry

35150-08-4
86 suppliers
$11.00/250mg

24424-99-5
866 suppliers
$13.50/25G
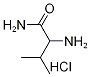
93169-29-0
23 suppliers
$165.00/1g

35150-08-4
86 suppliers
$11.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

35150-08-4
86 suppliers
$11.00/250mg

24424-99-5
866 suppliers
$13.50/25G
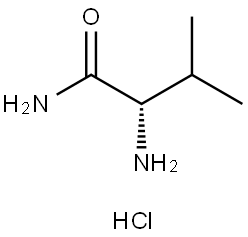
3014-80-0
284 suppliers
$13.43/1gm:

35150-08-4
86 suppliers
$11.00/250mg