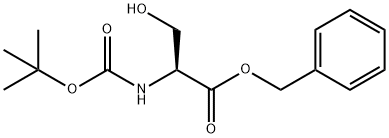
BOC-SER-OH synthesis
- Product Name:BOC-SER-OH
- CAS Number:59524-02-6
- Molecular formula:C15H21NO5
- Molecular Weight:295.33

3262-72-4

100-39-0

59524-02-6
GENERAL STEPS: To a stirred solution of N-Boc-L-serine (4.87 mmol) in DMF (100 mL) was added cesium carbonate (5.11 mmol) and stirring was continued for 30 minutes. Benzyl bromide (5.84 mmol) was then added and the reaction mixture was stirred for 12 hours. After completion of the reaction, the reaction mixture was diluted with ethyl acetate (25 mL) and washed sequentially with aqueous lithium bromide (3 × 15 mL), aqueous sodium bicarbonate (2 × 15 mL) and saturated saline (2 × 15 mL). The organic layer was dried with anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent. The crude product was purified by fast chromatography (eluent: petroleum ether/ether = 1:1) to afford Boc-L-serine benzyl ester (100% yield) as a white solid. rf = 0.26 (petroleum ether/ether = 1:1). To a stirred solution of N-Boc-L-serine (2.44 mmol) in DMF (50 mL) was added cesium carbonate (2.56 mmol) and stirring was continued for 30 min. Benzyl bromide (2.92 mmol) was then added and the reaction mixture was stirred for 12 hours. After completion of the reaction, the reaction mixture was diluted with ethyl acetate (15 mL) and washed sequentially with aqueous lithium bromide (3 × 10 mL), aqueous sodium bicarbonate (2 × 10 mL) and saturated saline (2 × 10 mL). The organic layer was dried with anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent. The crude product was purified by fast chromatography (eluent: petroleum ether/ether = 1:1) to give Boc-L-serine benzyl ester (100% yield) as a white solid. rf = 0.26 (petroleum ether/ether = 1:1).

3262-72-4
404 suppliers
$10.00/1g

100-39-0
438 suppliers
$10.00/10 g

59524-02-6
187 suppliers
$5.00/1g
Yield:59524-02-6 100%
Reaction Conditions:
Stage #1:(S)-N-(tert-butoxycarbonyl)serine with caesium carbonate in N,N-dimethyl-formamide for 0.5 h;
Stage #2:benzyl bromide in N,N-dimethyl-formamide for 12 h;Product distribution / selectivity;
Steps:
1
To a stirring solution of N-Boc- (L) -Serine (4.87 mmol) in DMF (100 mL) was added cesium carbonate (5.11 mmol) and stirring was continued 30 minutes. Benzyl bromide (5.84 mmol) was then added and the resulting solution was stirred 12 hours. The reaction mixture was then diluted with ethyl acetate (25 mL), washed with lithium bromide (3 x 15 mL), sodium bicarbonate (2 x 15 mL), and brine (2 x 15mL). The organic layer was dried over sodium sulfate. The solvent was then removed under reduced pressure and the resulting tan oil was purified by flash chromatography, using 1: 1 petroleum ether/diethyl ether, to afford the product (100%) as a white solid. Rf= 0. 26 (1: 1 petroleum ether/diethyl ether).; To a stirring solution of N-Boc- (L) -Serine (2.44 mmol) in DMF (50 mL) was added cesium carbonate (2. 56 mmol) and stirring was continued 30 minutes. Benzyl bromide (2. 92 mmol) was then added and the resulting solution was stirred 12 hours. The reaction mixture was then diluted with ethyl acetate (15 mL), washed with lithium bromide (3 x 10 mL), sodium bicarbonate (2 x 10 mL), and brine (2 x 10 mL). The organic layer was dried over sodium sulfate. The solvent was then removed under reduced pressure and the resulting tan oil was purified by flash chromatography, using 1: 1 petroleum ether/diethyl ether, to afford the product (100%) as a white solid. Rf= 0.26 (1: 1 petroleum ether/diethyl ether).
References:
UNIVERSITY OF VIRGINIA PATENT FOUNDATION WO2005/41899, 2005, A2 Location in patent:Page/Page column 94; 102-103
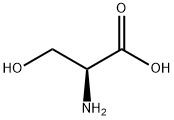
56-45-1
803 suppliers
$5.00/25g

24424-99-5
867 suppliers
$13.50/25G

100-39-0
438 suppliers
$10.00/10 g

59524-02-6
187 suppliers
$5.00/1g

24424-99-5
867 suppliers
$13.50/25G
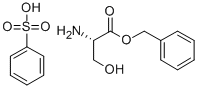
3695-68-9
15 suppliers
$57.29/5 g

59524-02-6
187 suppliers
$5.00/1g

24424-99-5
867 suppliers
$13.50/25G

59524-02-6
187 suppliers
$5.00/1g

24424-99-5
867 suppliers
$13.50/25G

1738-72-3
80 suppliers
$79.00/5g

59524-02-6
187 suppliers
$5.00/1g