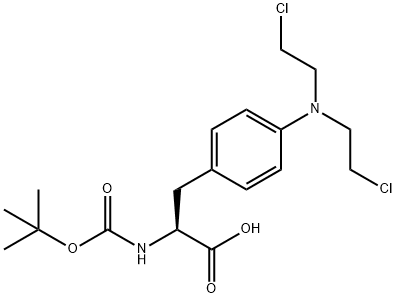
BOC-4-BIS(2-CHLOROETHYL)AMINO-L-PHENYLALANINE synthesis
- Product Name:BOC-4-BIS(2-CHLOROETHYL)AMINO-L-PHENYLALANINE
- CAS Number:79145-92-9
- Molecular formula:C18H26Cl2N2O4
- Molecular Weight:405.32

24424-99-5
869 suppliers
$13.50/25G
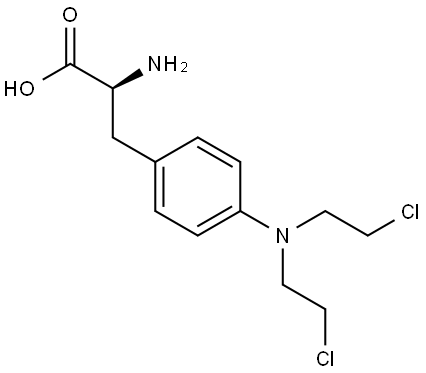
148-82-3
0 suppliers
$30.00/5mg

79145-92-9
6 suppliers
$212.00/100mg
Yield:79145-92-9 98%
Reaction Conditions:
in 1,4-dioxane;water at 0 - 20; for 17 h;
Steps:
2.i
First, t-Boc protected L-melphalan, 2 is synthesized by adding di-tert-butyl dicarbonate (196 mg, 0.89 mmol) to an ice-cold solution of melphalan (1-250 mg, 0.82 mmol) in a mixture of dioxane (2 mL), distilled water (1 mL), and 1N NaOH (1 mL). The mixture is stirred for 1 h at 0° C. and then for 16 h at room temperature. After the reaction is complete, the mixture is concentrated and ethyl acetate and distilled water are added. The pH of the mixture is adjusted to 2 with hydrochloric acid and the aqueous phase is then extracted with ethyl acetate (3 times with 15 ml). The combined organic phases are washed with distilled water and brine, dried over MgSO4, and the filtrate is concentrated under vacuum to yield compound 2 (330 mg, yield 98%).
References:
US2005/137141,2005,A1 Location in patent:Page/Page column 5; 6

24424-99-5
869 suppliers
$13.50/25G

79145-92-9
6 suppliers
$212.00/100mg
58632-95-4
277 suppliers
$7.00/5g

79145-92-9
6 suppliers
$212.00/100mg
