
Deucravacitinib synthesis
- Product Name:Deucravacitinib
- CAS Number:1609392-27-9
- Molecular formula:C20H22N8O3
- Molecular Weight:422.45
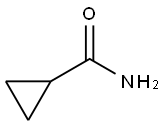
6228-73-5
238 suppliers
$5.00/1g
1609394-23-1
6 suppliers
inquiry

1609392-27-9
185 suppliers
inquiry
Yield:1609392-27-9 46%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);caesium carbonate;4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in 1,4-dioxane at 130; for 2.33333 h;Sealed tube;Inert atmosphere;
Steps:
52.2 Example
Step 2[00220j Intl3 (2.32 g, 6.16 mmol) and cyclopropanecarboxamide (1.048 g, 12.31 mmol) were dissolved in dioxane (62 mL) and Pd2(dba)3 (564 mg, 0.6 16 mmol), Xantphos (534 mg, 0.924 mmol) and cesium carbonate (4.01 g, 12.3 mmol) were added.The vessel was evacuated three times (backfilling with nitrogen) and then sealed and heated to 130 °C for 140 minutes. The reaction was filtered through CELITE (eluting with ethyl acetate) and concentrated (on smaller scale this material could then be purified using preparative HPLC). The crude product was adsorbed onto CELITE using dichloromethane, dried and purified using automated chromatography (100% EtOAc)provide example 52 (1.22 g, 46% yield). ‘H NMR (500MHz, chloroform-d) ? 10.99 (s,1H), 8.63 (s, 1H), 8.18 (s, 1H), 8.10 (d, J=0.5 Hz, 2H), 7.81 (dd, J7.9, 1.7 Hz, 1H), 7.51(dd, J7.9, 1.4 Hz, 1H), 7.33 - 7.20 (m, 7H), 4.01 (d, J0.3 Hz, 3H), 3.82 (s, 3H), 1.73 -1.60 (m, 1H), 1.16 - 1.06 (m, 2H), 0.97 - 0.84 (m, 2H). LC retention time 6.84 [N].MS(Ej m/z: 426 (MHj
References:
BRISTOL-MYERS SQUIBB COMPANY;MOSLIN, Ryan M.;WEINSTEIN, David S.;WROBLESKI, Stephen T.;TOKARSKI, John S.;KUMAR, Amit WO2014/74661, 2014, A1 Location in patent:Paragraph 00220

1609394-10-6
160 suppliers
inquiry

1609392-27-9
185 suppliers
inquiry
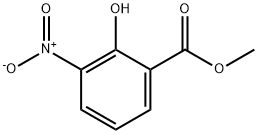
22621-41-6
191 suppliers
$10.00/1g

1609392-27-9
185 suppliers
inquiry
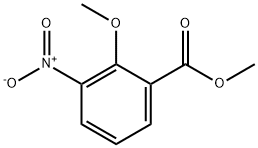
90564-26-4
91 suppliers
$90.00/100mg

1609392-27-9
185 suppliers
inquiry
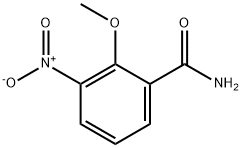
722538-98-9
30 suppliers
inquiry

1609392-27-9
185 suppliers
inquiry