
BENZYL PHENYL CARBONATE 97 synthesis
- Product Name:BENZYL PHENYL CARBONATE 97
- CAS Number:28170-07-2
- Molecular formula:C14H12O3
- Molecular Weight:228.24
1885-14-9

28170-07-2
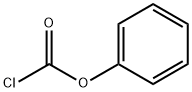
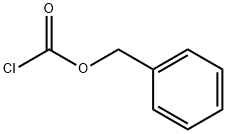
Synthesis of benzyl phenyl carbonate (Synthesis, 2002, 15, 2195-2202) Benzyl alcohol (10.8 g, 100.0 mmol) was dissolved in 100 mL of dichloromethane (CH2Cl2). After the solution was cooled to 0°C, phenyl chloroformate (15.7 g, 100 mmol) was added slowly and dropwise. The reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, 100 mL of water (H2O) was added, followed by washing twice with 100 mL of 2 M sulfuric acid (H2SO4). The organic phase was separated and dried over anhydrous sodium sulfate (Na2SO4). The solvent was removed by distillation under reduced pressure to give the colorless liquid product benzyl phenyl carbonate (22.5 g, 99% yield). The product was characterized by 1H NMR (CDCl3, 300 MHz): δ= 7.45-7.31 (m, 6H, arylhydrogen), 7.25-7.14 (m, 4H, arylhydrogen), 5.25 (s, 2H, benzylhydrogen).
124-38-9
132 suppliers
$175.00/23402

100-39-0
437 suppliers
$10.00/10 g

108-95-2
767 suppliers
$14.00/25g

28170-07-2
80 suppliers
$14.56/1G
Yield:28170-07-2 78%
Reaction Conditions:
with ethyl(8-diisopropylamino-3,6-dioxaoctyl)dimethylammonium bis{(trifluoromethyl)sulfonyl}azanide at 25; under 7500.75 Torr; for 72 h;Autoclave;Green chemistry;
Steps:
Experimental
General procedure: All the reactions were carried out in a 16 mL stainless-steelautoclave with stirring at 600 rpm and equipped with an automatic stirrer and temperature control system. In a typicalreaction procedure, IL (0.60 mmol, 1.2 equiv.), benzyl bromide(0.5 mmol, 85.5 mg, 1.0 equiv.), and alcohols (1.0 mmol, 2.0equiv.) were added into the autoclave successively. CO2 (1.0MPa) was charged into the reactor at room temperature. The autoclave was stirred at 25°C for an appropriate time. Afterreaction, the excess of CO2 was vented. Ethyl ether (2 mL) was added to the mixture, and the crude product was extracted intothe ether layer. The pure product was obtained after evaporation of solvent and excess starting materials.
References:
Goodrich, Peter;Gunaratne, H. Q. Nimal;Jin, Lili;Lei, Yuntao;Seddon, Kenneth R. [Australian Journal of Chemistry,2018,vol. 71,# 2-3,p. 181 - 185]

1885-14-9
348 suppliers
$10.00/1g

100-51-6
1444 suppliers
$5.00/100g

28170-07-2
80 suppliers
$14.56/1G

1885-14-9
348 suppliers
$10.00/1g

28170-07-2
80 suppliers
$14.56/1G
501-53-1
416 suppliers
$10.00/1g

108-95-2
767 suppliers
$14.00/25g

28170-07-2
80 suppliers
$14.56/1G