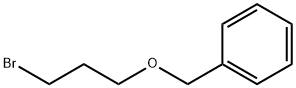
BENZYL 3-BROMOPROPYL ETHER synthesis
- Product Name:BENZYL 3-BROMOPROPYL ETHER
- CAS Number:54314-84-0
- Molecular formula:C10H13BrO
- Molecular Weight:229.11
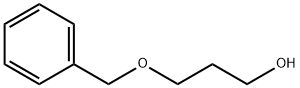
4799-68-2

54314-84-0
GENERAL STEPS: To a dry reaction flask equipped with a magnetic stirrer under nitrogen protection, copper catalyst (2 mg, 0.002 mmol, 0.01 equiv), 3-benzyloxy-1-propanol (0.20 mmol, 1.0 equiv), carbon tetrabromide (131.6 mg, 0.4 mmol, 2.0 equiv) and sodium bromide (41 mg. 0.40 mmol, 2.0 equiv). The reaction vial was sealed and anhydrous DMF (1.5 mL) was added using a syringe. The reaction mixture was stirred under the light of a purple LED (394 nm) for 24 hours. Upon completion of the reaction, the mixture was transferred to a dispensing funnel and extracted by adding ether (10 mL) and water (10 mL). The organic phase was separated and the aqueous phase was further extracted with ether (2 x 10 mL). The organic phases were combined and washed sequentially with saturated aqueous NaHCO3 solution, Na2S2O3 solution and brine, dried with anhydrous Na2SO4 and concentrated under reduced pressure. Finally, the target product 3-benzyloxypropyl bromide was purified by silica gel column chromatography (100% hexane).

4799-68-2
187 suppliers
$5.00/1g

54314-84-0
225 suppliers
$8.00/1g
Yield:54314-84-0 99%
Reaction Conditions:
with carbon tetrabromide;Cu(tmp)(BINAP)BF4;sodium bromide in N,N-dimethyl-formamide for 24 h;Catalytic behavior;UV-irradiation;Inert atmosphere;Appel Halogenation;Reagent/catalyst;Time;
Steps:
General procedure for the Appel reaction in batch
General procedure: To an open oven-dried reaction vial charged with a stir bar was added copper catalyst (2 mg,0.002 mmol, 0.01 equiv), the alcohol (0.20 mmol, 1.0 equiv), carbon tetrabromide (131.6 mg, 0.4mmol, 2.0 equiv) and sodium bromide (41 mg, 0.40 mmol, 2.0 equiv). The flask vial was capped,purged with a stream of nitrogen and dry DMF (1.5 mL) was added via syringe. The reactionmixture was stirred under purple LEDs (394 nm) for 24 h. The vessel was opened and the mixturewas poured into a separatory funnel containing Et2O (10 mL) and H2O (10 mL). The layers wereseparated, and the aqueous layer was extracted with Et2O (2 × 10 mL). The combined organiclayers were washed with sat. Na2S2O3 solution, brine, dried over Na2SO4 and concentrated invacuo. The residue was purified by chromatography (100 % hexanes)
References:
Minozzi, Clémentine;Grenier-Petel, Jean-Christophe;Parisien-Collette, Shawn;Collins, Shawn K. [Beilstein Journal of Organic Chemistry,2018,vol. 14,p. 2730 - 2736] Location in patent:supporting information

14593-43-2
127 suppliers
$18.00/1g

54314-84-0
225 suppliers
$8.00/1g

100-39-0
438 suppliers
$10.00/10 g

627-18-9
387 suppliers
$9.00/1g

54314-84-0
225 suppliers
$8.00/1g