
Benzaldehyde, 2-broMo-4-hydroxy synthesis
- Product Name:Benzaldehyde, 2-broMo-4-hydroxy
- CAS Number:22532-60-1
- Molecular formula:C7H5BrO2
- Molecular Weight:201.02

64-17-5
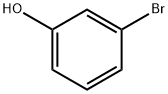
591-20-8

22532-60-1
The general procedure for the synthesis of 2-bromo-4-hydroxybenzaldehyde from ethanol and m-bromophenol was as follows: 30% aqueous sodium hydroxide solution (160 mL) was mixed with 3-bromophenol (10.04 g, 58 mmol) and heated to 70-75 °C. Subsequently, ethanol (5 mL) was added all at once and chloroform (28 mL, 0.35 mol) was added slowly and dropwise. After maintaining the reaction at 75 °C for 1 hour, the reaction mixture was continued to be stirred at this temperature for 3 hours. Upon completion of the reaction, the mixture was cooled to room temperature and acidified with 10N hydrochloric acid. Most of the by-products were removed by steam distillation. The residue was cooled and the precipitate was separated by filtration. The crude product was purified by silica gel column chromatography using hexane/ethyl acetate (2:1, v/v) as eluent to give 1.75 g of 2-bromo-4-hydroxybenzaldehyde in 15% yield.

123-08-0
956 suppliers
$5.00/10g

22532-60-1
93 suppliers
$29.00/1g
Yield:22532-60-1 88%
Reaction Conditions:
with 1,2-diphenyl-1,1,2,2-tetrahydroperoxyethane;hydrogen bromide in water;acetonitrile at 20; for 2.5 h;
Steps:
Bromination of anilines and phenol derivatives (Scheme 2, entry 8) General procedure
General procedure: To a solution of aniline/phenol (1 mmol) in CH3CN (4 mL), HBr and THPDPE (depending on the substrate as shown in Table 7) were added and the solution was stirred at room temperature. After the reaction was completed, Na2SO3 (3M, 1mL) was added to the stirring mixture followed by the addition of H2O (10 mL). The solution was stirred until the desired precipitates appeared. The products were filtered and more purification was carried out using silica- packed column chromatography (Hexane-EtOAc). All of the products were characterized on the basis of their melting points, IR, 1H NMR, and 13C NMR spectral analysis and compared with those reported
References:
Khosravi, Kaveh;Naserifar, Shirin [Tetrahedron,2018,vol. 74,# 45,p. 6584 - 6592] Location in patent:supporting information
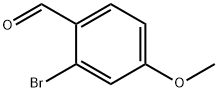
43192-31-0
136 suppliers
$8.00/1g

22532-60-1
93 suppliers
$29.00/1g

83521-93-1
4 suppliers
inquiry

22532-60-1
93 suppliers
$29.00/1g

36282-26-5
208 suppliers
$7.00/5g

22532-60-1
93 suppliers
$29.00/1g

106-44-5
502 suppliers
$14.00/5g

22532-60-1
93 suppliers
$29.00/1g