
9-Chloroacridine synthesis
- Product Name:9-Chloroacridine
- CAS Number:1207-69-8
- Molecular formula:C13H8ClN
- Molecular Weight:213.66
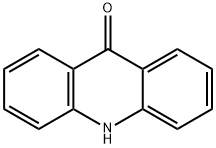
578-95-0

1207-69-8
The general procedure for the synthesis of 9-chloroacridine from acridinone was as follows: 9-acridinone (4.01 g, 21 mmol) was dissolved in phosphoryl chloride (105 mL, 1.13 mol), a magnetic stirrer was added, and the reaction was carried out at reflux for 12 hours. Upon completion of the reaction, the reaction solution was cooled to room temperature, slowly poured into crushed ice (650 g) and the pH was adjusted to a weak base with concentrated ammonium hydroxide. The resulting light brown solid was collected by filtration, washed well with water and subsequently dried in air for 24 hours. Purification by fast column chromatography (silica gel as stationary phase, eluent was a solvent mixture of 75% hexane, 20% THF and 5% triethylamine) gave 3.97 g (89% yield) of the target product, 9-chloroacridine, as fine yellow needle-like crystals. The obtained product was confirmed to be consistent with the sample prepared by Method A by TLC, melting point determination and spectral data analysis.
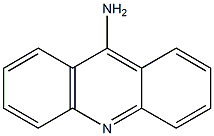
110166-26-2
1 suppliers
inquiry

1207-69-8
198 suppliers
$13.00/250mg
Yield:1207-69-8 36%
Reaction Conditions:
with pyrylium tetrafluoroborate;magnesium chloride in acetonitrile; for 16 h;Heating;Sealed tube;
Steps:
General procedure for the chlorination of amino heteroaromatic compounds
General procedure: Unless otherwise specified, an 18 ml screw-capped tube under normal atmosphere is charged with pyrylium tetrafluoroborate 1 (1.5 equiv.) and MgCl2 (2.0 equiv.). The starting material (1.0 equiv.) is then added and directly followed by CH3CN (0.1 M). The resulting mixture is then stirred 5 minutes at 25 °C and then 16 hours at 120 °C. The reaction is allowed to cool to 25 °C. The crude mixture is partitioned between water and EtOAc. The aqueous layer is extracted with EtOAc (3 × 10 ml). The combined organic layers are dried over Na2SO4, concentrated to dryness and purified on silica gel to afford the desired product.
References:
Ghiazza, Clément;Faber, Teresa;Gómez-Palomino, Alejandro;Cornella, Josep [Nature Chemistry,2022,vol. 14,# 1,p. 78 - 84]
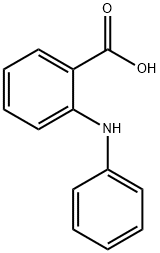
91-40-7
334 suppliers
$10.00/1g

1207-69-8
198 suppliers
$13.00/250mg

578-95-0
310 suppliers
$5.00/1g

1207-69-8
198 suppliers
$13.00/250mg
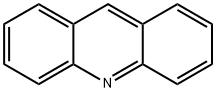
260-94-6
172 suppliers
$20.00/1g

1207-69-8
198 suppliers
$13.00/250mg
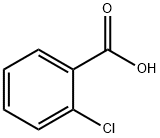
118-91-2
675 suppliers
$14.00/25g

1207-69-8
198 suppliers
$13.00/250mg