
8-BROMO-2H-ISOQUINOLIN-1-ONE synthesis
- Product Name:8-BROMO-2H-ISOQUINOLIN-1-ONE
- CAS Number:475994-60-6
- Molecular formula:C9H6BrNO
- Molecular Weight:224.05

475994-58-2

475994-60-6
The general procedure for the synthesis of 8-bromoisoquinolin-1(2H)-one from 8-bromoisoquinoline 2-oxide was as follows: 8-bromoisoquinoline 2-oxide (630 mg, 2.8 mmol) was suspended in N,N-dimethylformamide (DMF, 10 mL), and treated with trifluoroacetic anhydride (4 mL). The reaction mixture was stirred overnight at room temperature until the solution was clarified. Subsequently, the reaction solution was concentrated under reduced pressure to remove the solvent. The residue was neutralized with dilute aqueous sodium carbonate and extracted several times with a 5% methanol/dichloromethane mixture. The organic phases were combined, washed with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography, eluting with a gradient of 0-10% methanol in dichloromethane solution to give a white solid product (180 mg, 29% yield). Mass spectrometric analysis (positive ion electrospray mode) showed m/z 225 [M+H]+.

475994-58-2
6 suppliers
inquiry

475994-60-6
90 suppliers
$34.00/100 mg
Yield: 52.5%
Reaction Conditions:
with acetic anhydride in dichloromethane for 3 h;Reflux;
Steps:
169.2 Step- 2: Synthesis of 8-bromo-2H-isoquinolin-1-one
The 8-bromo-2-oxido-isoquinolin-2-ium (80 g, 357.06 mmol) was suspended in acetic anhydride (729.03 g, 7.14 mmol, 675.03 mL) and the resulting mixture was heated at reflux for 3 hr then allowed to cool to room temp.The Ac2O was removed by distillation under reduced pressure to yield a solid residue that was suspended in an aqueous solution of NaOH (2 M, 600 mL).The resulting mixture was heated at 100 °C for 1 h then allowed to cool to RT.The pH of the resulting solution was adjusted to pH 6 by addition of an aqueous solution of citric acid and the mixture was extracted with ethyl acetate.The combined organic layers were dried over sodium sulphate and evaporated under reduced pressure to give the crude product 8-bromo-2H-isoquinolin-1-one (70g, 52.50% Yield).LCMS (ES+) =224.0 [M+H] +.
References:
C4 THERAPEUTICS, INC.;HENDERSON, James, A.;HE, Minsheng;GOOD, Andrew, Charles;PHILIPPS, Andrew, J. WO2020/210630, 2020, A1 Location in patent:Page/Page column 676-677
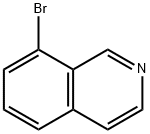
63927-22-0
267 suppliers
$7.00/250mg

475994-60-6
90 suppliers
$34.00/100 mg
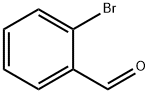
6630-33-7
426 suppliers
$9.00/10g

475994-60-6
90 suppliers
$34.00/100 mg
![Ethanamine, N-[(2-bromophenyl)methylene]-2,2-dimethoxy-](/CAS/20210111/GIF/405161-29-7.gif)
405161-29-7
0 suppliers
inquiry

475994-60-6
90 suppliers
$34.00/100 mg