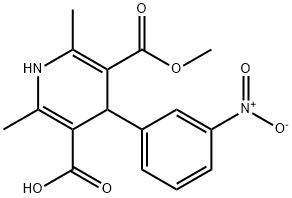
5-(Methoxycarbonyl)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid synthesis
- Product Name:5-(Methoxycarbonyl)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid
- CAS Number:74936-72-4
- Molecular formula:C16H16N2O6
- Molecular Weight:332.31
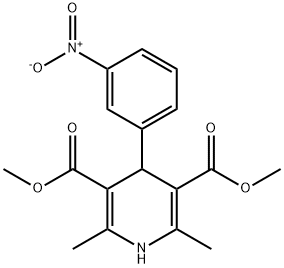
21881-77-6

74936-72-4
General procedure for the synthesis of monomethyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-4-(3-nitrophenyl)pyridine-3,5-dicarboxylate from dimethyl 2,6-dimethyl-4-(3-nitrophenyl)pyridine-3,5-dicarboxylate: (3) Preparation of 5-methoxycarbonyl-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid Dimethyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate (0.01 mol) was mixed with 150 mL of methanol. Saturated aqueous NaOH solution (9 g, 0.225 mol) was slowly added to the mixture under stirring conditions. The reaction mixture was vigorously stirred at 70 °C for 6 hours. Upon completion of the reaction, about 80 mL of methanol was removed by evaporation under reduced pressure. 200 mL of water was added to the residue and unreacted feedstock was removed by filtration. The filtrate was adjusted to pH about 2.5 with 1 mol/L hydrochloric acid, at which point a yellow solid precipitated, which was filtered and dried to give a khaki colored powder. The resulting powder was recrystallized from methanol to give the title product 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)pyridine-3,5-dicarboxylic acid monomethyl ester as a yellow solid (1.9 g, 57.2% yield).
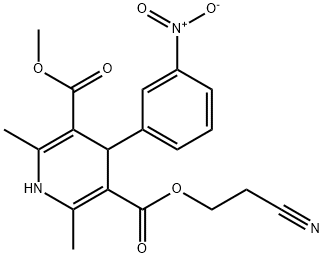
75130-24-4
38 suppliers
inquiry

74936-72-4
268 suppliers
$15.00/5g
Yield:74936-72-4 86.8%
Reaction Conditions:
with water;sodium hydroxide at 20;Reagent/catalyst;
Steps:
1.3; 2.3
(3) Intermediate 2 (1800g),750 g of a 3% aqueous sodium hydroxide solution was added to the reaction.The reaction is carried out at room temperature for 2 to 3 hours; and then extracted with 10000 g of dichloromethane;The aqueous phase was acidified to pH = 1-2 with 10% hydrochloric acid. Filtered, dried,It has a pale yellow solid. The obtained solid was recrystallized from methanol to give Intermediate 3 (1346 g).The yield was 86.8%.
References:
CN109111431,2019,A Location in patent:Paragraph 0042; 0046; 0056; 0060

21881-77-6
126 suppliers
$8.00/1g

74936-72-4
268 suppliers
$15.00/5g

21881-77-6
126 suppliers
$8.00/1g
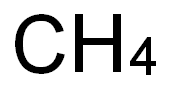
7440-44-0
348 suppliers
$12.00/25g

74936-72-4
268 suppliers
$15.00/5g
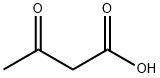
541-50-4
56 suppliers
inquiry

99-61-6
661 suppliers
$5.00/10g
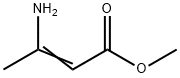
14205-39-1
517 suppliers
$6.00/25g

74936-72-4
268 suppliers
$15.00/5g

99-61-6
661 suppliers
$5.00/10g

74936-72-4
268 suppliers
$15.00/5g