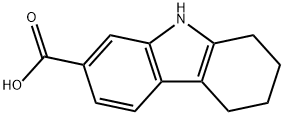
2,3,4,9-tetrahydro-1H-carbazole-7-carboxylic acid(SALTDATA: FREE) synthesis
- Product Name:2,3,4,9-tetrahydro-1H-carbazole-7-carboxylic acid(SALTDATA: FREE)
- CAS Number:729613-71-2
- Molecular formula:C13H13NO2
- Molecular Weight:215.25

108-94-1
577 suppliers
$12.00/50g

38235-71-1
144 suppliers
$41.00/5g

729613-71-2
23 suppliers
$45.00/10mg
Yield:729613-71-2 39%
Reaction Conditions:
with hydrogenchloride in water;acetic acid; for 3 h;Reflux;
Steps:
2 Step 2: Preparation of 2,3,4,9-tetrahydro-1 H-carbazole-7-carboxylic acid
To a stirred suspension of 3-hydrazinylbenzoic acid (200 mg, 1 .32 mmol) in acetic acid was added cyclohexanone (129 mg, 1 .32 mmol) and cone. HCI. The mixture was heated at reflux for 3 hours and cooled to room temperature. The resultant was concentrated directly in vacuum to remove acetic acid, the residue was washed with water and extracted with ethyl acetate, and the organic layer was extracted with 2 N NaOH solution. The alkaline layers were made acidic by the addition of 2 N HCI, extracted with diethyl ether, dried and concentrated to give a crude product, which was purified by column chromatography on silica gel to give 2,3,4,9-tetrahydro-1 - - carbazole-7-carboxylic acid as a pale solid (1 10 mg, 39%). 1 H NMR (400 MHz, DMSO-d6): δ 12.36 (s, 1 H), 1 1.04 (s, 1 H), 7.88 (s, 1 H), 7.55 (d, J = 9.2 Hz, 1 H), 7.38 (d, J = 8.4 Hz, 1 H), 2.73 (t, J = 4.8 Hz, 2H), 2.64 (t, J = 4.8 Hz, 2H), 1 .84-1 .81 (m, 4H).
References:
WO2016/8010,2016,A1 Location in patent:Page/Page column 38; 39

108-94-1
577 suppliers
$12.00/50g

38235-71-1
144 suppliers
$41.00/5g

729613-71-2
23 suppliers
$45.00/10mg

784143-99-3
2 suppliers
inquiry

99-05-8
549 suppliers
$14.00/5g
822-87-7
197 suppliers
$35.00/10g

729613-71-2
23 suppliers
$45.00/10mg