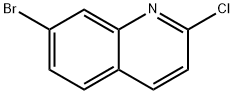
7-BROMO-2-CHLORO-QUINOLINE synthesis
- Product Name:7-BROMO-2-CHLORO-QUINOLINE
- CAS Number:99455-15-9
- Molecular formula:C9H5BrClN
- Molecular Weight:242.5

99465-10-8

99455-15-9
General procedure for the synthesis of 7-bromo-2-chloroquinoline from 7-bromoquinolin-2(1H)-one: To an inert reactor equipped with a mechanical stirrer, a reflux condenser, a charging funnel and a caustic scrubber were added 7-bromo-2-hydroxyquinoline (1.22 kg, 5.45 mol), dichloromethane (9.706 kg) and dimethylformamide (0.276 kg). Thionyl chloride (0.973 kg, 8.17 mol, 1.5 eq.) was added slowly and dropwise. The reaction mixture was gradually heated to reflux and kept at reflux until the reaction was complete (about 2 hours). After completion of the reaction, the mixture was cooled to 20-25 °C, USP grade purified water (3.663 kg) was added and stirred. Subsequently, a solution of potassium carbonate (0.828 kg) dissolved in USP grade purified water (0.828 kg) was slowly added with stirring. Stirring was stopped after 15 minutes and left to stratify. After confirming that the pH of the aqueous layer was greater than 7, the dichloromethane layer was separated and washed with USP grade purified water (3.663 kg). USP grade purified water (7.326 kg) was added to the dichloromethane solution and the dichloromethane was removed by distillation until the product precipitated from the aqueous layer. The reactor was cooled to 20-25 °C, the product was collected by filtration and washed with USP grade purified water (2 x 1.22 kg). The product was dried in a vacuum oven at 40-50 °C to give the final 7-bromo-2-chloroquinoline (1.154 kg, 78.8% yield). The product was analyzed by NMR and the results were consistent with the expected structure.

99465-10-8
114 suppliers
$22.00/100mg

99455-15-9
133 suppliers
$12.00/100mg
Yield: 78.8%
Reaction Conditions:
Stage #1:7-bromo-quinolin-2-ol with thionyl chloride in dichloromethane;N,N-dimethyl-formamide for 2 h;Heating / reflux;
Stage #2: in dichloromethane;water;N,N-dimethyl-formamide; pH=> 7
Steps:
6
Example 6 Preparation of Compound of Formula (VIII): 7-Bromo-2-chloroquinoline. An inerted reactor equipped with a mechanical stirrer, a reflux condenser, an addition funnel and caustic scrubber was charged with 7-bromo-2-hydroxyquinoline (1.22 Kg, 5.45 mole), dichloromethane (9.706 Kg), and dimethylformamide (0.276 Kg) followed by a slow addition of thionyl chloride (0.973 Kg, 8.17 mole, 1.5 equiv.). The reaction mixture was then gradually heated to reflux. The reflux was continued until solution is achieved (about 2 hours). The reaction mixture was then cooled to 20 to 25°C, and USP purified water (3.663 Kg) was added and stirred. Then to the reactor was gradually added with stirring a solution of potassium carbonate (0.828 Kg) in USP purified water (0.828 Kg). After 15 minutes, the stirring was stopped and the layers were allowed to separate. The pH of aqueous layer was confirmed greater than 7. The dichloromethane solution was separated and washed with USP purified water (3.663 Kg). USP purified water (7.326 Kg) was then added to the dichloromethane solution, and the dichloromethane was distilled off until the product precipitated out of the aqueous layer. The reactor was cooled to 20 to 25°C. The product was filtered and washed USP purified water (2 x 1.22 Kg). The product was dried in a vacuum oven at 40 to 5O0C to obtain 1.154 Kg of desired product. Yield: 78.8%. NMR: Compatible with product.
References:
SANOFI-AVENTIS U.S. LLC WO2009/2955, 2008, A1 Location in patent:Page/Page column 10

917341-34-5
0 suppliers
inquiry

99455-15-9
133 suppliers
$12.00/100mg
99465-09-5
60 suppliers
$65.00/25mg

99465-09-5
60 suppliers
$65.00/25mg

99455-15-9
133 suppliers
$12.00/100mg

99455-13-7
84 suppliers
$55.00/10 mg

99465-10-8
114 suppliers
$22.00/100mg

99465-09-5
60 suppliers
$65.00/25mg

99455-15-9
133 suppliers
$12.00/100mg

99455-13-7
84 suppliers
$55.00/10 mg

99465-10-8
114 suppliers
$22.00/100mg

99455-15-9
133 suppliers
$12.00/100mg