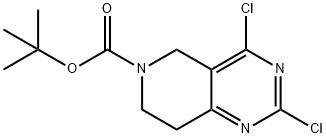
TERT-BUTYL 2,4-DICHLORO-7,8-DIHYDROPYRIDO[4,3-D]PYRIMIDINE-6(5H)-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 2,4-DICHLORO-7,8-DIHYDROPYRIDO[4,3-D]PYRIMIDINE-6(5H)-CARBOXYLATE
- CAS Number:635698-56-5
- Molecular formula:C12H15Cl2N3O2
- Molecular Weight:304.17
![2,4-DICHLORO-5,6,7,8-TETRAHYDROPYRIDO[3,4-D]PYRIMIDINE](/CAS/GIF/726697-13-8.gif)
726697-13-8

24424-99-5
![TERT-BUTYL 2,4-DICHLORO-7,8-DIHYDROPYRIDO[4,3-D]PYRIMIDINE-6(5H)-CARBOXYLATE](/CAS/GIF/635698-56-5.gif)
635698-56-5
2,4-Dichloro-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine (270 mg, 1.33 mmol) and di-tert-butyl dicarbonate (348 mg, 1.6 mmol) were used as the starting material, and 2,4-dichloro-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine was dissolved in dichloromethane (30 mL) at 0 °C. Triethylamine (200 mg, 2.0 mmol) was then added and the reaction mixture was stirred at room temperature for 16 hours. After completion of the reaction, the organic layer was separated by diluting the reaction mixture with dichloromethane (30 mL) and water (30 mL). The aqueous phase was further extracted with dichloromethane (30 mL x 2). All organic layers were combined, washed with brine and dried with anhydrous sodium sulfate. After filtration to remove the desiccant, the solvent was removed by concentration under reduced pressure. The resulting crude product was purified by fast column chromatography using a solvent mixture of hexane and ethyl acetate to afford N-BOC-2,4-dichloro-5,7,8-trihydropyrido[4,3-D]pyrimidine (300 mg, 74% yield). Mass spectrometry analysis (LRMS, M+H+): calculated value 305.17; measured value 305.24.
![2,4-dichloro-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine hydrochloride](/CAS2/GIF/1208901-69-2.gif)
1208901-69-2
54 suppliers
$38.00/100mg

24424-99-5
867 suppliers
$13.50/25G
![TERT-BUTYL 2,4-DICHLORO-7,8-DIHYDROPYRIDO[4,3-D]PYRIMIDINE-6(5H)-CARBOXYLATE](/CAS/GIF/635698-56-5.gif)
635698-56-5
134 suppliers
$24.00/100mg
Yield: 100%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 0.333333 h;Product distribution / selectivity;
Steps:
4.1
2,4-Dichloro-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine hydrochloride prepared according to Reference Example 1 (2.00 g) and triethylamine (2.80 mL, 2.4 equivalents) were dissolved in dichloromethane (40 mL), and the solution was mixed with di-tert-butyl dicarbonate (2.29 mL, 1.2 equivalents), followed by stirring at room temperature for twenty minutes. The reaction mixture was sequentially washed with water, a saturated aqueous sodium bicarbonate solution and brine, followed by extraction with chloroform. The organic layer was dried over anhydrous magnesium sulfate, and the solvent was distilled off, to thereby yield tert-butyl 2,4-dichloro-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine-6-car boxylate (3.0g, in a quantitative yield).
References:
KYOWA HAKKO KOGYO CO., LTD. EP1552842, 2005, A1 Location in patent:Page/Page column 316
![2,4-DICHLORO-5,6,7,8-TETRAHYDROPYRIDO[3,4-D]PYRIMIDINE](/CAS/GIF/726697-13-8.gif)
726697-13-8
37 suppliers
$312.00/250mg

24424-99-5
867 suppliers
$13.50/25G
![TERT-BUTYL 2,4-DICHLORO-7,8-DIHYDROPYRIDO[4,3-D]PYRIMIDINE-6(5H)-CARBOXYLATE](/CAS/GIF/635698-56-5.gif)
635698-56-5
134 suppliers
$24.00/100mg
![6-BENZYL-2,4-DICHLORO-5,6,7,8-TETRAHYDROPYRIDO[4,3-D]PYRIMIDINE](/CAS/GIF/778574-06-4.gif)
778574-06-4
121 suppliers
$104.00/1g
![TERT-BUTYL 2,4-DICHLORO-7,8-DIHYDROPYRIDO[4,3-D]PYRIMIDINE-6(5H)-CARBOXYLATE](/CAS/GIF/635698-56-5.gif)
635698-56-5
134 suppliers
$24.00/100mg
![6-BENZYL-5,6,7,8-TETRAHYDRO-1H-PYRIDO[4,3-D]PYRIMIDINE-2,4-DIONE](/CAS/GIF/135481-57-1.gif)
135481-57-1
83 suppliers
$18.00/250mg
![TERT-BUTYL 2,4-DICHLORO-7,8-DIHYDROPYRIDO[4,3-D]PYRIMIDINE-6(5H)-CARBOXYLATE](/CAS/GIF/635698-56-5.gif)
635698-56-5
134 suppliers
$24.00/100mg

57611-47-9
0 suppliers
$47.00/1g
![TERT-BUTYL 2,4-DICHLORO-7,8-DIHYDROPYRIDO[4,3-D]PYRIMIDINE-6(5H)-CARBOXYLATE](/CAS/GIF/635698-56-5.gif)
635698-56-5
134 suppliers
$24.00/100mg