
(6-methoxypyridin-3-yl)methanol synthesis
- Product Name:(6-methoxypyridin-3-yl)methanol
- CAS Number:58584-63-7
- Molecular formula:C7H9NO2
- Molecular Weight:139.15
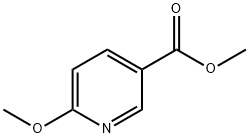
26218-80-4

58584-63-7
The general procedure for the synthesis of (6-methoxypyridin-3-yl)methanol from methyl 6-methoxynicotinate is as follows: Preparation Example 1: Synthesis of (6-methoxypyridin-3-yl)methanol (2) Methyl-6-methoxynicotinate (1) (650 g, 3.89 mol) was dissolved in tert-butyl methyl ether (MTBE, 6.5 L) and cooled in an ice bath under nitrogen protection. Sodium bis(2-methoxyethoxy) aluminum hydride (65% toluene solution, 1.45 kg, 4.67 mol) was slowly added to the reaction system over 1.5 hours. After addition, stirring was continued for 20 minutes. An aqueous 3.5N sodium hydroxide solution (2.6L) was slowly added while keeping the reaction temperature below 15°C. The reaction mixture was warmed up to 32°C and stirred for 45 minutes. After completion of the reaction, the organic layer was separated and the aqueous layer was extracted again with MTBE (2.3 L). The organic layers were combined and concentrated to dryness under reduced pressure. Toluene (1.3 L) was added to the residue and azeotropic distillation was performed. This azeotropic distillation process was repeated three times to give 597 g of the title compound as a light yellow oil (100% yield). 1H-NMR (CDCl3) δ (ppm): 8.11 (1H, d, J=2.4Hz), 7.62 (1H, dd, J=2.4Hz, 8.8Hz), 6.75 (1H, d, J=8.8Hz), 4.62 (2H, s), 3.93 (3H, s).

26218-80-4
163 suppliers
$7.00/1g

58584-63-7
103 suppliers
$60.00/5g
Yield: 100%
Reaction Conditions:
with sodium bis(2-methoxyethoxy)aluminium dihydride in tert-butyl methyl ether;toluene at 0; for 1.63333 h;
Steps:
1 Synthesis of (6-methoxypyridin-3-yl)methanol (2)
Preparation Example 1 Synthesis of (6-methoxypyridin-3-yl)methanol (2) To a solution of methyl-6-methoxynicotinate (1) (650 g, 3.89 mol) in t-butyl methyl ether (hereinafter abbreviated as "MTBE") (6.5 L) cooled in an ice bath was added sodium bis(2-methoxyethoxy)aluminum hydride (65% solution in toluene, 1.45 kg, 4.67 mol) under a nitrogen atmosphere over a period of 1.3 hours. After stirring for 20 minutes, a 3.5 N aqueous solution of sodium hydroxide (2.6 L) was added to the reaction mixture while keeping the temperature 15° C. or below. The reaction mixture was stirred at 32° C. for 45 minutes and then the organic layer was separated and the aqueous layer was re-extracted with MTBE (2.3 L). The organic layers were combined and concentrated under reduced pressure to dryness, and then toluene (1.3 L) was added to the residue and azeotropic distillation was carried out. Azeotropic distillation with toluene (1.3 L) was repeated three times to give 597 g of the title compound as a pale yellow oil (yield 100%). 1H-NMR (CDCl3) δ (ppm): 8.11 (1H, d, J=2.4 Hz), 7.62 (1H, dd, J=2.4 Hz, 8.8 Hz), 6.75 (1H, d, J=8.8 Hz), 4.62 (2H, s), 3.93 (3H, s)
References:
Eisai Co., Ltd. US2006/235225, 2006, A1 Location in patent:Page/Page column 3

65873-72-5
284 suppliers
$5.00/250mg

58584-63-7
103 suppliers
$60.00/5g
50-00-0
893 suppliers
$10.00/25g
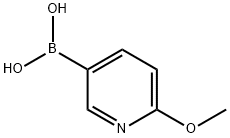
163105-89-3
388 suppliers
$10.00/1g

58584-63-7
103 suppliers
$60.00/5g

66572-55-2
178 suppliers
$15.00/5g

58584-63-7
103 suppliers
$60.00/5g
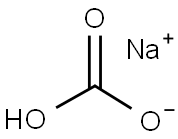
144-55-8
1372 suppliers
$10.00/25g

3099-50-1
22 suppliers
inquiry
7440-05-3
509 suppliers
$17.69/1G

58584-63-7
103 suppliers
$60.00/5g