
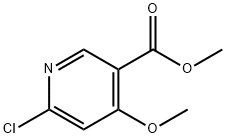
6-Chloro-4-methoxypyridine-3-carbaldehyde synthesis
- Product Name:6-Chloro-4-methoxypyridine-3-carbaldehyde
- CAS Number:1256823-05-8
- Molecular formula:C7H6ClNO2
- Molecular Weight:171.58
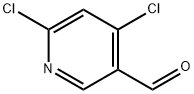
1060811-62-2
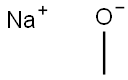
124-41-4

1256823-05-8
Sodium methanolate (0.068 g, 1.25 mmol) was added batchwise to a stirred solution of 4,6-dichloropyridine-3-carbaldehyde (0.2 g, 1.14 mmol) in methanol (10 mL) at 0 °C. The reaction mixture was slowly warmed to room temperature (about 5 hours) and stirring was continued at room temperature for 16 hours. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure and the residue was dissolved in dichloromethane. Insoluble solids were removed by filtration and the filtrate was purified by fast silica gel column chromatography with an elution gradient of 0 to 50% ethyl acetate in heptane solution. The purified grades were collected and concentrated under reduced pressure to give 6-chloro-4-methoxynicotinaldehyde (0.111 g, 57% yield) as a white solid.1H NMR (500 MHz, CDCl3, 22 °C) δ 4.02 (3H, s), 6.97 (1H, s), 8.69 (1H, s), 10.37 (1H, d). Mass spectrum (ESI+): m/z 172 [M+H]+.

1060811-62-2
135 suppliers
$15.00/100mg
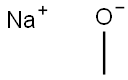
124-41-4
726 suppliers
$12.00/25g

1256823-05-8
47 suppliers
$95.00/250mg
Yield:1256823-05-8 57%
Reaction Conditions:
in methanol at 0 - 25; for 21 h;Inert atmosphere;
Steps:
7 Preparation of 6-chloro-4-methoxynicotinaldehyde
Sodium methoxide (0.068 g, 1.25 mmol) was added portionwise to a stirred solution of 4,6-dichloronicotinaldehyde (0.2 g, 1.14 mmol) in MeOH (10 mL) cooled to 0°C. The reaction was allowed to warm to room temperature (over 5 hours) and stirred at roomtemperature for 16 hours. The reaction was concentrated in vacuo and then dissolved in DCM. Solids were removed by filtration and the filtrate was purified by flash silica chromatography, elution gradient 0 to 50% EtOAc in heptane. Pure fractions were evaporated to dryness to afford 6-chloro-4-methoxynicotinaldehyde (0.111 g, 57%) as a white solid. ‘H NMR (500 MHz, CDC13, 22 °C) 4.02 (3H, s), 6.97 (1H, s), 8.69 (1H, s),10.37 (1H, d). nz/z: ES+ [M+H]+ 172.
References:
WO2018/19793,2018,A1 Location in patent:Page/Page column 126; 127
84332-02-5
66 suppliers
$65.00/100mg

1256823-05-8
47 suppliers
$95.00/250mg
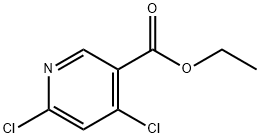
40296-46-6
334 suppliers
$6.00/1g

1256823-05-8
47 suppliers
$95.00/250mg
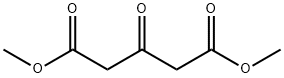
1830-54-2
409 suppliers
$9.00/10g

1256823-05-8
47 suppliers
$95.00/250mg

79398-27-9
124 suppliers
$11.00/250mg

1256823-05-8
47 suppliers
$95.00/250mg