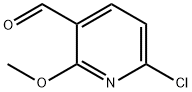
6-chloro-2-methoxynicotinaldehyde synthesis
- Product Name:6-chloro-2-methoxynicotinaldehyde
- CAS Number:95652-81-6
- Molecular formula:C7H6ClNO2
- Molecular Weight:171.58

17228-64-7
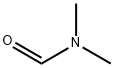
68-12-2

95652-81-6
GENERAL STEPS: 2-Chloro-6-methoxypyridine (5 g, 34.82 mmol) was dissolved in tetrahydrofuran (THF, 100 mL) under dry argon atmosphere and cooled to -78 °C. Tert-butyl lithium (tBuLi, 1.7 M, 18.5 mL, 31.34 mmol) was slowly added dropwise, keeping the temperature at -78 °C, and the reaction was stirred for 1 hour. Subsequently, N,N-dimethylformamide (DMF, 92.2 g, 31.34 mmol) was slowly added at the same temperature and stirring was continued for 2 hours. After completion of the reaction, the reaction was quenched with acetic acid and the mixture was poured into ice-cold water. The aqueous phase was alkalized with saturated sodium bicarbonate (NaHCO3) solution and subsequently extracted with ethyl acetate (EtOAc, 2 x 500 mL). The organic layers were combined, dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography with the eluent being a mixed solvent of petroleum ether and ethyl acetate (10:1, v/v) to afford 6-chloro-2-methoxypyridine-3-carboxaldehyde as a white solid (3.8 g, 65% yield). The mass spectrum (ES+) showed m/z 172 [M+H]+; elemental analysis (C7H6ClNO2) was consistent with the theoretical value; 1H NMR (300 MHz, CDCl3) δ 10.17 (s, 1H), 8.07 (d, J=8.04Hz, 1H), 7.03 (d, J=8.04Hz, 1H), 3.79 (s, 3H).

55304-73-9
164 suppliers
$6.00/1g
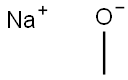
124-41-4
725 suppliers
$12.00/25g

95652-81-6
93 suppliers
$23.00/100mg
Yield:95652-81-6 71.3%
Reaction Conditions:
with methanol at 55; for 3 h;
Steps:
184.A.A1
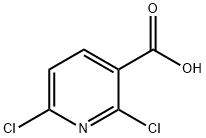
2,6-Dichloronicotinaldehyde (1 g, 5.68 mmol) was diluted with sodium methoxide (11.4 mL, 5.68 mmol) (solution in methanol) and heated to 55°C. After stirring for 3 hours, the reaction was loaded directly onto a silica gel column and eluted with 5% ethyl acetate/hexanes to 50% ethyl acetate/hexanes to yield 6-chloro-2- methoxynicotinaldehyde (0.695 g, 4.05 mmol, 71.3 % yield).
References:
WO2010/75200,2010,A1 Location in patent:Page/Page column 164

17228-64-7
314 suppliers
$6.00/5g

95652-81-6
93 suppliers
$23.00/100mg

55304-73-9
164 suppliers
$6.00/1g

95652-81-6
93 suppliers
$23.00/100mg
38496-18-3
380 suppliers
$6.00/1g
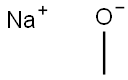
124-41-4
725 suppliers
$12.00/25g

95652-80-5
59 suppliers
$97.00/100mg

95652-81-6
93 suppliers
$23.00/100mg

67-56-1
785 suppliers
$9.00/25ml

55304-73-9
164 suppliers
$6.00/1g

95652-81-6
93 suppliers
$23.00/100mg
