
6-Bromonicotinic acid synthesis
- Product Name:6-Bromonicotinic acid
- CAS Number:6311-35-9
- Molecular formula:C6H4BrNO2
- Molecular Weight:202.01

3510-66-5

6311-35-9
General procedure for the synthesis of 6-bromonicotinic acid from 2-bromo-5-methylpyridine: 2-bromo-5-methylpyridine (100 g, 0.291 mol) was dissolved in 1000 mL of water and Aliquat 336 (2 mL) was added as a phase transfer catalyst. Subsequently, potassium permanganate (251 g, 0.797 mol) was added slowly over 1 hr. The reaction mixture was stirred at 110 °C for 30 min and continued for 1 h to ensure complete reaction. Upon completion of the reaction, the mixture was filtered through diatomaceous earth while hot and the diatomaceous earth was washed with hot water. The filtrate was concentrated to half the original volume under reduced pressure. To the concentrate was added 48% hydrobromic acid (about 300 mL), and the precipitated 6-bromonicotinic acid crystals were filtered, washed with water and dried to give 52 g of white crystals in 44% yield. The product was characterized by 1H-NMR (CDCl3) with chemical shift δ (ppm) of 7.64 (1H, d, J = 8.0 Hz), 8.08 (1H, d, J = 8.0 Hz), 9.03 (1H, s).

3510-66-5
482 suppliers
$5.00/5g

6311-35-9
381 suppliers
$15.00/1g
Yield:6311-35-9 44%
Reaction Conditions:
with potassium permanganate;Aliquat 336 in water at 110; for 2.5 h;
Steps:
22.1 (Step 1) 6-Bromonicotinic acid
After dissolving 2-bromo-5-picoline (100 g, 0.291 mol) in 1000 ml of water, Aliquat336 (2 ml) was added, and then potassium permanganate (251 g, 0.797 mol) was gradually added over a period of 1 hour and 30 minutes while stirring at 110°C. This mixture was further stirred for an hour, the reaction mixture was filtered through celite without cooling and washed with water, and the filtrate was concentrated to approximately half volume under reduced pressure. After adding 48% hydrobromic acid (~300 ml), the precipitated crystals were filtered, washed with water and dried to yield the title compound (white crystals 52 g, 44%).1H-NMR(CDCl3)δ(ppm) 7.64(1H,d,J=8.0Hz), 8.08(1H,d,J=8.0Hz), 9.03(1H,s).
References:
EP1391451,2004,A1 Location in patent:Page 143

26218-78-0
235 suppliers
$6.00/1g

6311-35-9
381 suppliers
$15.00/1g
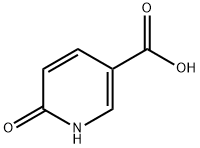
5006-66-6
485 suppliers
$13.43/1gm:

6311-35-9
381 suppliers
$15.00/1g
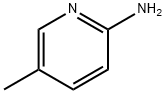
1603-41-4
551 suppliers
$7.00/25g

6311-35-9
381 suppliers
$15.00/1g

122306-01-8
169 suppliers
$6.00/250mg

6311-35-9
381 suppliers
$15.00/1g