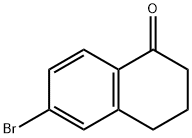
6-BROMO-TETRAL-1-ON synthesis
- Product Name:6-BROMO-TETRAL-1-ON
- CAS Number:66361-67-9
- Molecular formula:C10H9BrO
- Molecular Weight:225.08
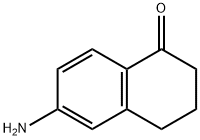
3470-53-9

66361-67-9
The general procedure for the synthesis of 6-bromo-3,4-dihydronaphthalen-1(2H)-one from 6-amino-1,2,3,4-tetrahydro-1-naphthalenone was as follows: an aqueous solution (10 mL) of NaNO2 (2.35 g, 34 mmol) was added slowly and dropwise at 0°C to a 6-amino-1,2,3,4-tetrahydronaphthalen-1-one (5.0 g, 31 mmol) in a 25% HBr (16 mL) solution. Subsequently, the resulting suspension was transferred to a stirred mixture of 48% HBr (30 mL) of CuBr (8.9 g, 62 mmol) at 0 °C. The reaction mixture was gradually warmed to room temperature and stirred continuously for 1 hour. After completion of the reaction, the mixture was extracted with EtOAc and the organic phase was dried and concentrated with Na2SO4. The residue was purified by silica gel column chromatography (elution gradient: 0%-60% EtOAc/Hex) to afford 5.6 g (80% yield) of 6-bromo-3,4-dihydronaphthalen-1(2H)-one as a light yellow oil.1H NMR (400 MHz, CDCl3) δ: 2.10-2.16 (2H, m), 2.64 (2H, t, J = 6.4 Hz), 2.94 (2H, t, J = 6.0 Hz), 7.42 (1H, s), 7.44 (1H, s), 7.87 (1H, d, J = 8.9 Hz). Mass spectral analysis: [M + H]+ calculated value C10H9BrO: 225,227; measured value: 225,227.

3470-53-9
220 suppliers
$6.00/250mg

66361-67-9
170 suppliers
$50.00/100mg
Yield:66361-67-9 83%
Reaction Conditions:
Stage #1: 6-amino-1-tetralonewith hydrogen bromide;sodium nitrite in water; for 0.25 h;
Stage #2: with copper(I) bromide in water; for 0.25 h;
Steps:
6-bromo-3,4-dihydronaphthalen-1(2H)-one 3
To the solution of 2 (5 g and 31 mmol) in a mixture of hydrobromic acid (50 mL and47%) and water (50 mL) cooled by a brine/ice bath, a solution of sodium nitrite (2.5 gand 36 mmol) in water (20 mL) was added dropwise. The resulting mixture was stirredfor 15 min before a solution of cuprous bromide (5.14 g and 36 mmol) in hydrobromicacid (20 mL and 47%) was added dropwise. The resulting mixture was then stirred for15 min. The aqueous layers were washed with ethyl acetate (3 15 mL). The organic layerswere combined, dried over Na2SO4, filtered and evaporated. The resulting yellow oil waspuried by chromatography
References:
Chen, Baiyu;Fu, Wei;Li, Jianqi;Li, Wei;Li, Xinwei;Xie, Peng;Zhang, Junjie;Zhang, Wenbo;Zhu, Mengdan [Molecules,2022,vol. 27,# 7,art. no. 2173]

19936-14-2
70 suppliers
$43.00/100mg

66361-67-9
170 suppliers
$50.00/100mg

228256-58-4
9 suppliers
inquiry

66361-67-9
170 suppliers
$50.00/100mg

524-38-9
514 suppliers
$5.00/25g

35333-26-7
47 suppliers
$52.00/100mg

66361-67-9
170 suppliers
$50.00/100mg
3470-50-6
234 suppliers
$6.00/1g

66361-67-9
170 suppliers
$50.00/100mg