
6-BROMO-2-METHOXY-PYRIDIN-3-YLAMINE synthesis
- Product Name:6-BROMO-2-METHOXY-PYRIDIN-3-YLAMINE
- CAS Number:89466-18-2
- Molecular formula:C6H7BrN2O
- Molecular Weight:203.04

20265-38-7

89466-18-2
2-Methoxypyridin-3-amine (39.4 g) was dissolved in N,N-dimethylformamide (200 mL) and the solution was cooled to -30 °C. A solution of N,N-dimethylformamide (100 mL) of N-bromosuccinimide (62.1 g) was slowly added dropwise with stirring. After the dropwise addition, the reaction was continued with stirring for 30 minutes. Subsequently, the reaction mixture was poured into water and extracted with chloroform. The organic layers were combined and washed sequentially with saturated sodium sulfite solution, water and brine. The organic layer was dried with anhydrous sodium sulfate, filtered and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent ratio: hexane:ethyl acetate=9:1→4:1) to afford 6-bromo-2-methoxypyridin-3-amine as a yellow powder (51.9 g, 80% yield). The structure of the product was confirmed by 1H NMR (300 MHz, CDCl3) and mass spectrometry: 1H NMR δppm 3.64-3.84 (m, 2H), 3.98 (s, 3H), 6.78 (dd, J=7.9,1.0 Hz, 1H), 6.87 (d, J=7.9 Hz, 1H); MS (+): 203 [M+H]+.
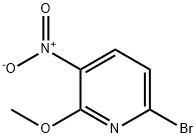
58819-77-5
46 suppliers
$45.00/10mg

89466-18-2
176 suppliers
inquiry
Yield:89466-18-2 100%
Reaction Conditions:
with iron;ammonium chloride in ethanol;water at 80; for 1 h;Inert atmosphere;
Steps:
1.2 Step 2: Synthesis of 6-bromo-2-methoxypyridine-3-amine
At room temperature, 6-bromo-2-methoxy-3-nitropyridine (9.50 g, 38.62 mmol),Iron powder (11.50g, 193.11mmol), ammonium chloride (10.80g, 193.11mmol) and ethanol (190ml),Water (95 ml) was protected by nitrogen, the temperature was raised to 80 ° C, and the reaction was terminated in 1 h.The reaction solution was filtered with celite, the filtrate was concentrated, water (950 ml) was added, EA (380 ml x 2) was extracted, and the organic phases were combined.Saturated saline (200 ml) was added for washing, and then concentrated to obtain 8.28 g of a black oily liquid with a yield of 100%.
References:
CN110642838,2020,A Location in patent:Paragraph 0113; 0119-0121
39856-57-0
296 suppliers
$10.00/1g
124-41-4
727 suppliers
$12.00/25g

89466-18-2
176 suppliers
inquiry

20265-38-7
262 suppliers
$6.00/250mg

89466-18-2
176 suppliers
inquiry

55304-80-8
185 suppliers
$10.00/1g

89466-18-2
176 suppliers
inquiry
626-05-1
511 suppliers
$9.00/10g

89466-18-2
176 suppliers
inquiry