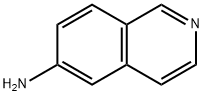
6-AMINOISOQUINOLINE synthesis
- Product Name:6-AMINOISOQUINOLINE
- CAS Number:23687-26-5
- Molecular formula:C9H8N2
- Molecular Weight:144.17
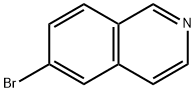
34784-05-9

23687-26-5
General procedure for the synthesis of 6-aminoisoquinoline from 6-bromoisoquinoline: 17.2 g of 6-bromoisoquinoline (see WO 2008/077553), 200 mL of 28% ammonia solution and 10.8 g of copper (II) sulfate pentahydrate were placed in an autoclave and sealed. The mixture was stirred and reacted at 190 °C for 6 hours. After completion of the reaction, it was cooled to room temperature and the reaction solution was poured into 250 mL of 10% aqueous sodium hydroxide solution and extracted with ethyl acetate (100 mL each time, 5 times). The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated. The resulting crude product was suspended with dichloromethane and filtered to give 10.2 g of light brown crystalline 6-aminoisoquinoline in 85% yield.1H-NMR spectrum (CDCl3, δ ppm): 5.54 (broad single peak, 2H), 6.58 (single peak, 1H), 7.00 (double peak, J = 9.0 Hz, 1H), 7.35 (double peak, J = 5.5 Hz, 1H), 7.75 (double peak, J = 5.5 Hz, 1H). 7.75 (double peak, J = 9.0 Hz, 1H), 8.32 (double peak, J = 5.5 Hz, 1H), 8.98 (single peak, 1H).

34784-05-9
326 suppliers
$5.00/1g

23687-26-5
203 suppliers
$16.00/100mg
Yield:23687-26-5 85%
Reaction Conditions:
with ammonium hydroxide;copper(ll) sulfate pentahydrate in water at 190; for 6 h;
Steps:
1 1
Reference Example 1 Synthesis of 6-aminoisoquinoline (Reference Compound 1) 6-bromoisoquinoline that weighed 17.2 g (see WO 2008/077553), 200 mL of 28% ammonia water and 10.8 g of copper (II) sulfate pentahydrate were put into the autoclave and tightly sealed, and the mixture was then stirred at 190° C. for 6 hours. After cooling to room temperature, the reaction solution was poured into 250 mL of a 10% aqueous sodium hydroxide solution, followed by extraction with ethyl acetate (100 mL*5). The extract was dried over anhydrous sodium sulfate, filtered, and then concentrated. The obtained crude product was suspended in dichloromethane and then filtered to obtain 10.2 g of the compound of interest as a light brown crystal (85%). 1H-NMR spectrum (CDCl3, δ ppm): 5.54 (br s, 2H), 6.58 (s, 1H), 7.00 (d, J=9.0 Hz, 1H), 7.35 (d, J=5.5 Hz, 1H), 7.75 (d, J=9.0 Hz, 1H), 8.32 (d, J=5.5 Hz, 1H), 8.98 (s, 1H)
References:
D. WESTERN THERAPEUTICS INSTITUTE, INC US2012/35159, 2012, A1 Location in patent:Page/Page column 8
70538-57-7
17 suppliers
inquiry

23687-26-5
203 suppliers
$16.00/100mg

7651-82-3
143 suppliers
$8.00/100mg

1336-21-6
733 suppliers
$14.56/250ML

23687-26-5
203 suppliers
$16.00/100mg

7651-82-3
143 suppliers
$8.00/100mg

23687-26-5
203 suppliers
$16.00/100mg
39585-32-5
50 suppliers
$45.00/50mg

23687-26-5
203 suppliers
$16.00/100mg