
2-METHYL-1,2,3,4-TETRAHYDRO-ISOQUINOLINE-3-CARBOXYLIC ACID synthesis
- Product Name:2-METHYL-1,2,3,4-TETRAHYDRO-ISOQUINOLINE-3-CARBOXYLIC ACID
- CAS Number:54329-54-3
- Molecular formula:C11H13NO2
- Molecular Weight:191.23

67123-97-1

54329-54-3
GENERAL STEPS: Preparation of Example 3 2-methyl-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid 0.364 g (2.07 mmol) of 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid was dissolved in 25 mL of formic acid. To this solution was added 2.5 mL of 37% formaldehyde solution. The resulting mixture was heated to reflux for about 17 hours. Upon completion of the reaction, the mixture was cooled and concentrated under reduced pressure to give a gummy substance. The gummy was dissolved in 3.0 mL of water and the pH was adjusted with hydrochloric acid to 2. Subsequently, purification was carried out using ion exchange chromatography (Dowex 50x-8, 100 mesh) to give 0.21 g of yellow powdery product in 53% yield. Product characterization data: 1H NMR (DMSO-d6): δ 2.56 (s, 3H), 3.01 (m, 2H), 3.48 (t, 1H), 3.9 (dd, 2H), 7.05 (m, 1H), 7.13 (m, 3H). MS (FD): m/e 192 (M+2).

67123-97-1
137 suppliers
$9.00/1g

54329-54-3
61 suppliers
$18.00/100mg
Yield:54329-54-3 53%
Reaction Conditions:
with hydrogenchloride;formaldehyd in formic acid;water;
Steps:
3 2-Methyl-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
PREPARATION 3 2-Methyl-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid A solution of 0.364 g (2.07 mmol) of 3-carboxy-1,2,3,4-tetrahydroisoquinoline in 25 mL of formic acid was combined with 2.5 mL of 37% formaldehyde. The resulting mixture was allowed to reflux for approximately 17 hours. After cooling, the mixture was contentrated under reduced pressure to provide a gum. This gum was dissolved in 3.0 mL of water, adjusted to a pH 2 using hydrochloric acid and then purified using ion-exchange chromatography (Dowex 50x-8, 100 meshcation) to provide 0.21 g of a yellow powder. Yield: 53% 1 H NMR (DMSO-d6): δ2.56 (S, 3H), 3.01 (m, 2H), 3.48 (t, 1H), 3.9 (dd, 2H) 7.05 (m, 1H), 7.13 (m, 3H). MS (FD): m/e 192 (M+2).
References:
US5491166,1996,A
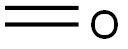
50-00-0
892 suppliers
$10.00/25g

67123-97-1
137 suppliers
$9.00/1g

54329-54-3
61 suppliers
$18.00/100mg