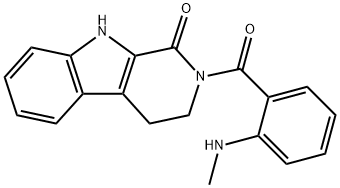
2-[2-(Methylamino)benzoyl]-3,4-dihydro-β-carboline-1(2H)-one synthesis
- Product Name:2-[2-(Methylamino)benzoyl]-3,4-dihydro-β-carboline-1(2H)-one
- CAS Number:526-43-2
- Molecular formula:C19H17N3O2
- Molecular Weight:319.36
Yield:526-43-2 27%
Reaction Conditions:
Stage #1: 1,2,3,4-tetrahydronorharman-1-onewith trichlorophosphate in tetrahydrofuran at 60; for 3 h;Inert atmosphere;
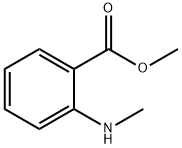
Stage #2: Methyl N-methylanthranilate in tetrahydrofuran at 75; for 96 h;Inert atmosphere;Further stages;
Steps:
1.1 4.1.1. 2-[2-(Methylamino)benzoyl]-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-1-one (2b).
To a stirred solution of 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-1-one (190 mg, 1.01 mmol, 1.0 equiv) in dry THF (15 mL), POCl3 (0.13 mL, 1.42 mmol, 1.4 equiv) was added and the mixture was stirred under Ar atmosphere for 3 h at 60 °C. Then, N-methylanthranilate (280 mg, 1.69 mmol, 1.7 equiv) was added, the temperature increased to 75 °C and the mixture stirred for 96 h. The solution was cooled to rt, CH2Cl2 and 2M NaOH were added (pH=13). The water layer was removed and the remaining orange colored organic layer was acidified with 2M HCl (pH=2). By this the color disappeared and water was added. The organic phase was removed and 2M NaOH was added to the water phase. The water phase was extracted three times with CH2Cl2 (3*30 mL) and dried over Na2SO4. The solvent was evaporated and the remaining brownish oil was kept in the freezer. Upon warming it to rt light yellowish crystals formed. The crystals were filtered off and washed tree times with diethyl ether. A yellow solid was formed during washing. This was dried and then washed as above. This yielded an orange solid (89.4 mg, 0.28 mmol, 27%). Rf=0.20 (SiO2, 100% ethyl acetate). Mp=170.7-173.0 °C. 1H NMR (400 MHz, CDCl3, 300 K): δ=10.12 (s, 1H, NHindole), 7.63-7.61 (m, 1H, Ar-HE-ring), 7.44 (dd, J=8.0, 1.6 Hz, 1H, Ar-HA-ring), 7.38-7.34 (m, 1H, Ar-HA-ring), 7.30-7.23 (m, 1H, Ar-HE-ring), 7.19 (d, J=5.1 Hz, 1H, NHCH3) 7.17-7.13 (m, 1H, Ar-HE-ring), 7.09 (d, J=8.3 Hz, 1H, Ar-HE-ring), 6.77 (dd, J=8.6, 1.0 Hz, 1H, Ar-HA-ring), 6.53-6.49 (m, 1H, Ar-HA-ring), 4.19 (t, J=6.5 Hz, 2H, NCH2CH2), 3.20 (t, J=6.5 Hz, 2H, NCH2CH2), 2.95 (d, J=4.8 Hz, 3H, CH3) ppm. 13C{1H} NMR (101 MHz, CDCl3, 300 K): δ=175.98 (s, C=O), 162.27 (s, C=O), 151.15 (s, Cquart.), 151.05 (s, Cquart.), 138.70 (s, Cquart.), 134.66 (s, Ar-C), 132.50 (s, Ar-C), 126.24 (s, Ar-C), 124.83 (s, Cquart.), 122.94 (s, Cquart.), 120.71 (s, Ar-C), 120.67 (s, Ar-C), 115.90 (s, Cquart.), 114.73 (s, Ar-C), 113.16 (s, Ar-C), 111.22 (s, Ar-C), 47.64 (s, NCH2CH2), 29.89 (s, CH3), 21.29 (s, NCH2CH2) ppm. IR: ν=3403w, 3264m, 2811w, 2371w, 2357w, 2349w, 1662s, 1605m, 1574m, 1551m, 1511m, 1487m, 1424w, 1395m, 1368w, 1321m, 1287m, 1265w, 1243w, 1231m, 1202m, 1174m, 1128w, 1107w, 1093w, 1067w, 1047m, 1003w, 993w, 980w, 946m, 892m, 851w, 803w, 776w, 768m, 747s, 740s, 712m, 676w, 659m cm-1. HPLC: Synergi 4U fusion-RP (15*0.46 cm), water/methanol (30-95%), 0.1% formic acid, 1.00 mL/min, 20 °C, tR=4.537 min, purity=95.30%. Mass: calcd for [M+H]+ (C19H18N3O2) requires m/z: 320.14 (open), 302.13 (closed); found: 302.20.
References:
Wehle, Sarah;Espargaró, Alba;Sabaté, Raimon;Decker, Michael [Tetrahedron,2016,vol. 72,# 20,p. 2535 - 2543]

118-48-9
440 suppliers
$5.00/5G
![2-[2-(Methylamino)benzoyl]-3,4-dihydro-β-carboline-1(2H)-one](/CAS/20150408/GIF/526-43-2.gif)
526-43-2
11 suppliers
inquiry
61-54-1
509 suppliers
$12.00/5G
![2-[2-(Methylamino)benzoyl]-3,4-dihydro-β-carboline-1(2H)-one](/CAS/20150408/GIF/526-43-2.gif)
526-43-2
11 suppliers
inquiry
118-92-3
0 suppliers
$23.00/250g
![2-[2-(Methylamino)benzoyl]-3,4-dihydro-β-carboline-1(2H)-one](/CAS/20150408/GIF/526-43-2.gif)
526-43-2
11 suppliers
inquiry

10328-92-4
149 suppliers
$18.00/1g
![2-[2-(Methylamino)benzoyl]-3,4-dihydro-β-carboline-1(2H)-one](/CAS/20150408/GIF/526-43-2.gif)
526-43-2
11 suppliers
inquiry
![2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-1-one](/CAS/GIF/17952-82-8.gif)