
3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin-2(1H)-one synthesis
- Product Name:3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin-2(1H)-one
- CAS Number:503615-03-0
- Molecular formula:C15H17N3O4
- Molecular Weight:303.31
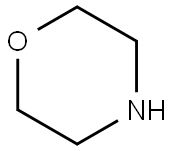
110-91-8
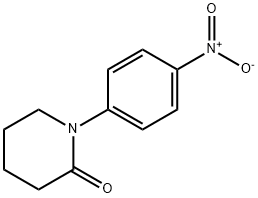
38560-30-4

503615-03-0
The reaction mixture was quenched with 1-(4-nitrophenyl)-2-piperidone (33 g, 0.15 mol) and phosphorus pentachloride (109 g, 0.52 mol) by slowly pouring into 500 mL of ice water. The aqueous layer was separated and the aqueous phase was extracted with chloroform (3 x 100 mL). The organic phases were combined and the reaction mixture was extracted with ethyl acetate. Subsequently, the combined organic phases were washed once with 500 mL of saturated sodium chloride solution and dried with anhydrous magnesium sulfate. The desiccant was removed by filtration and the filtrate was concentrated under reduced pressure to give 57 g of a yellow solid. The solid (57 g) was placed in a reaction flask, morpholine (307 mL) was added, slowly heated to reflux, and the reaction was maintained for 2 hours. Upon completion of the reaction, it was cooled to room temperature and the morpholine hydrochloride solid precipitated. 600 mL of water was added to precipitate the product, and the yellow solid was collected by filtration and dried to give 30 g of the target compound 5,6-dihydro-3-(4-morpholinyl)-1-(4-nitrophenyl)-2(1H)-pyridinone in a total yield of 66% for the two-step reaction.

110-91-8
707 suppliers
$9.00/1g

881386-01-2
144 suppliers
inquiry

503615-03-0
344 suppliers
$31.00/1g
Yield:503615-03-0 90.4%
Reaction Conditions:
at 120; for 2 h;Inert atmosphere;
Steps:
1 Preparation of 1d
The 1c (19.0g, 65.7mmol) was dissolved in morpholine (100ml) to obtain a reaction mixture,reaction mixture at reflux for 2 hours 120 °C , reaction progress was followed by thin layer chromatography, the reaction was completed, vacuum distillation most morpholine was removed, the residue as a yellow solid which was washed with water stirred 1 hour, filtered, the filter cake washed three times, air-dried and dried 24 hours give 1d (18.0g, yellow solid), yield: 90.4%
References:
CN104650072,2016,B Location in patent:Paragraph 0122; 0125; 0135; 0136

110-91-8
707 suppliers
$9.00/1g

38560-30-4
174 suppliers
$11.00/250mg

503615-03-0
344 suppliers
$31.00/1g

100-01-6
65 suppliers
$11.19/25G

503615-03-0
344 suppliers
$31.00/1g

1575-61-7
377 suppliers
$14.00/1g

503615-03-0
344 suppliers
$31.00/1g

1039914-85-6
57 suppliers
inquiry

503615-03-0
344 suppliers
$31.00/1g