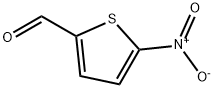
5-NITROTHIOPHENE-2-CARBOXALDEHYDE synthesis
- Product Name:5-NITROTHIOPHENE-2-CARBOXALDEHYDE
- CAS Number:4521-33-9
- Molecular formula:C5H3NO3S
- Molecular Weight:157.15
98-03-3

4521-33-9

57500-53-5
General procedure for the synthesis of 5-nitrothiophene-2-carbaldehyde and 4-nitrothiophene-2-carbaldehyde from 2-thiophenecarboxaldehyde: Step 1: Synthesis of 4-nitrothiophene-2-carbaldehyde (1) Fuming nitric acid (40 mL) was mixed with concentrated sulfuric acid (31 mL) and slowly added to a cooled solution of thiophene-2-carbaldehyde (20 g, 17.8 mmol) in concentrated sulfuric acid (4 mL). The reaction was carried out in an ice-salt bath and stirring was continued for 5 min after addition. Subsequently, the reaction was quenched by the addition of ice water and the mixture was extracted with ether. The combined ether extracts were washed sequentially with saturated sodium bicarbonate solution and brine, dried over anhydrous magnesium sulfate, filtered and concentrated to give a brown oily crude product. 1H NMR analysis of the crude product showed a mixture of 4-nitrothiophene-2-carboxaldehyde and 5-nitrothiophene-2-carboxaldehyde in the ratio of about 40:60. Separation of the mixture by column chromatography (eluent: 30-50% dichloromethane/hexanes) gave 4-nitrothiophene-2-carboxaldehyde in 40% yield as a light yellow solid.1H NMR (CDCl3) δ (ppm). 9.95 (s, 1H), 8.63 (s, 1H), 8.27 (s, 1H).

14289-24-8
9 suppliers
inquiry

4521-33-9
165 suppliers
$10.00/5g
Yield:4521-33-9 80%
Reaction Conditions:
with hydrogenchloride in methanol;water at 65; for 8 h;
Steps:
1.2; 1.3; 2.2; 2.3; 3.2; 3.3
(2) In a 250 mL four-neck flask, add 16 g of water and 71 g of methanol. Under stirring, add 7.5 g of the wet 5-nitrothiophen-2-ylmethylene diacetate obtained in step (1), and keep Under stirring conditions, 7.2g hydrochloric acid was added dropwise; refluxed at 65°C for 8 hours to prepare a reaction solution; the resulting reaction solution was naturally cooled to 30°C, 40 mL of water was added, stirred, and cooling continued to precipitate solids and filtered to obtain 5-nitrothiophene-2-carbaldehyde 6g;(3) In a 250mL four-necked flask, add the result of step (2) 6g of 5-nitrothiophene-2-carboxaldehyde, 15.8g of methanol, heated to 65°C, refluxed for 1h to prepare a reaction liquid; under stirring conditions, add 6.6g of n-hexane to the obtained reaction liquid, and cool to -5°C The solids are separated out, filtered and dried to obtain 5-nitro-2-thiophenecarboxaldehyde 4g, purity 99.3%, yield 80%.
References:
CN112939929,2021,A Location in patent:Paragraph 0035; 0037-0039; 0041-0043; 0045-0046

20898-85-5
35 suppliers
$45.00/50mg

4521-33-9
165 suppliers
$10.00/5g

63011-97-2
16 suppliers
inquiry

4521-33-9
165 suppliers
$10.00/5g

20898-83-3
0 suppliers
inquiry

4521-33-9
165 suppliers
$10.00/5g