
5'-EthylcarboxaMido-2',3'-isopropylidene Adenosine synthesis
- Product Name:5'-EthylcarboxaMido-2',3'-isopropylidene Adenosine
- CAS Number:39491-53-7
- Molecular formula:C15H20N6O4
- Molecular Weight:348.3571
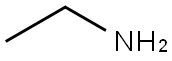
75-04-7
403 suppliers
$10.00/20mg

39491-49-1
0 suppliers
inquiry

39491-53-7
17 suppliers
$89.50/25mg
Yield:-
Reaction Conditions:
in dichloromethane at 0; for 1 h;
Steps:
3
Example 3-Synthesis of 2', 3'-O-Isopropylideneadenosine-5'- ethyluronamide [73] The 2', 3-O-ISOPROPYLIDENEADENOSINE-5-URONIC acid (Example 2,9. 4 g, 0.03 mol) is added quickly in small aliquots to thionyl chloride (30 ml) chilled to 0 °C, then 5 drops of DMF are added. The mixture is heated to 50 °C and after 1 hour 30 minutes the solvent is removed under reduced pressure and the residue is washed several times using diethyl ether. The residue constituting the acid chloride is dissolved in methylene chloride (100 ml), the solution is chilled to 0 °C, and a solution of ethylamine (39.43 ml) in methylene chloride (70 ml) is added slowly. The mixture is kept at 0 °C for 1 hour (TLC ethyl acetate/methylene chloride/methanol 8: 1.5 : 0.5). It is then washed sequentially with aqueous NAHCO3 and saline solution. The organic phase is dried with NA2SO4, evaporated, and the residue is recrystallized from a mixture of methylene chloride/diethyl ether. The product is collected as a pale yellow solid (6.7 g, 66% yield with respect to the 2', 3-O-ISOPROPYLIDENEADENOSINE-5- uronic ACID/51% yield with respect to the adenosine). [74] M. p: 213 °C (lit. 225-229 °C). LH-NMR (CDCL3) : 8 0.85-0. 92 (t, 3H, J=8); 1.39 (s, 3H); 1.63 (s, 3H); 3.06-3. 14 (m, 2H, J=2, J=6); 4.71 (s, 1H); 5.39- 5.40 (m, 2H, J=2); 5.82 (bs, 2H); 6.07 (s, 1H); 6.90 (m, 1H); 7.86 (s, 1H); 8.31 (s, 1H). Analyzed for CLSH20N6°4-
References:
WO2005/28489,2005,A2 Location in patent:Page/Page column 26

75-04-7
403 suppliers
$10.00/20mg

19234-66-3
31 suppliers
$185.00/250mg

39491-53-7
17 suppliers
$89.50/25mg

19234-66-3
31 suppliers
$185.00/250mg

39491-53-7
17 suppliers
$89.50/25mg

75-04-7
403 suppliers
$10.00/20mg

159647-45-7
0 suppliers
inquiry

39491-53-7
17 suppliers
$89.50/25mg