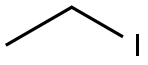
5-ETHYL-CYCLOPENTA-1,3-DIENE synthesis
- Product Name:5-ETHYL-CYCLOPENTA-1,3-DIENE
- CAS Number:22516-13-8
- Molecular formula:C7H10
- Molecular Weight:94.15
Yield:22516-13-8 82.6%
Reaction Conditions:
with tetrabutylammomium bromide;sodium hydroxide in water at 20; for 6 h;Reagent/catalyst;
Steps:
2 Example 2 (R = ethyl group; synthesis of ethylcyclopentadiene (Method (2))
0.328 g (0.365 mmol) of tetrabutylammonium bromide, 2.7 m (36.5 mmol) of ethyl bromide and 3.5 ml (43 mmol) of cyclopentadiene were added to a flask having an internal volume of 100 ml equipped with a dropping funnel and a thermometer And the mixture was stirred at room temperature. Next, 6 ml (48 mmol) of 8 mol / l sodium hydroxide aqueous solution was dropped and reacted for 6 hours with stirring. After completion of the reaction, the reaction solution was separated, the organic layer was separated, and the obtained organic layer was washed with water. When the obtained organic layer was analyzed by gas chromatography, the formation rate of ethylcyclopentadiene was 82.6%.
References:
JP2015/110581,2015,A Location in patent:Paragraph 0031