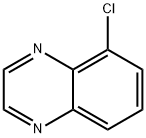
5-Chloroquinoxaline synthesis
- Product Name:5-Chloroquinoxaline
- CAS Number:62163-09-1
- Molecular formula:C8H5ClN2
- Molecular Weight:164.59

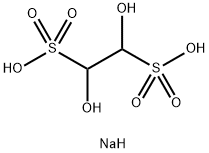
131543-46-9
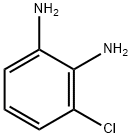
21745-41-5

62163-09-1
GENERAL METHODS: 3-Chlorophenyl-1,2-diamine (2.0 mmol) was mixed with the compound (CAS:131543-46-9, 2.0 mmol) and sulfated polyborate (10 wt%) was added. The reaction mixture was placed in an oil bath at 100 °C to stir the reaction and the progress of the reaction was monitored by thin layer chromatography (TLC). After completion of the reaction, the mixture was cooled to room temperature and the reaction was quenched with water. The organic phase was separated by extraction of the mixture with ethyl acetate (EtOAc). The crude product can be purified by recrystallization from ethanol or using silica gel column chromatography with silica as stationary phase and ethyl acetate: petroleum ether as mobile phase. The resulting 5-chloroquinoxaline, a known compound, was identified by determination of its melting point and 1H NMR and 13C NMR spectra, which were confirmed by comparing the spectral data with literature values.
Yield:62163-09-1 97%
Reaction Conditions:
with 10 wtpercent sulfated polyborate in neat (no solvent) at 100; for 0.05 h;Green chemistry;
Steps:
2.3 General procedure for the synthesis of quinoxaline derivatives
General procedure: To a mixture of substituted o-phenylenediamines derivative(2.0 mmol) and 1,2-diketone / α-hydroxy ketone (2.0 mmol),was added sulfated polyborate (10 wt%). The reaction mixture was stirred at 100 °C in an oil bath. The reaction was monitored by thin layer chromatography (TLC). After completion of the reaction, the mixture was cooled to room temperature and quenched by water. The resultant product was filtered/extracted with EtOAc to get the product. Crude products were either recrystallized from ethanol or purified by column chromatography using silica as the stationary phase and EtOAc: pet. ether as mobile phase. The products obtained were known compounds and were identified by melting point and 1H and 13C NMR spectroscopy. The spectral data were compared with the literature values.
References:
Indalkar, Krishna S;Khatri, Chetan K;Chaturbhuj, Ganesh U [Journal of Chemical Sciences,2017,vol. 129,# 2,p. 141 - 148]
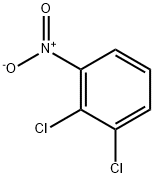
3209-22-1
20 suppliers
$10.00/1g

62163-09-1
49 suppliers
inquiry
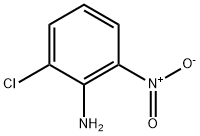
769-11-9
148 suppliers
inquiry

62163-09-1
49 suppliers
inquiry

6935-29-1
28 suppliers
$144.00/250mg
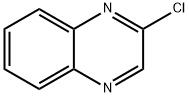
1448-87-9
164 suppliers
$10.00/500mg
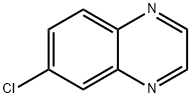
5448-43-1
76 suppliers
$15.00/1g

62163-09-1
49 suppliers
inquiry

1196-57-2
332 suppliers
$6.00/10g