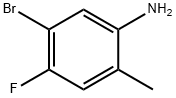
5-BROMO-4-FLUORO-2-METHYLANILINE synthesis
- Product Name:5-BROMO-4-FLUORO-2-METHYLANILINE
- CAS Number:627871-16-3
- Molecular formula:C7H7BrFN
- Molecular Weight:204.04
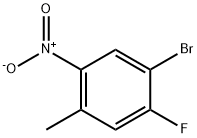
224185-19-7

627871-16-3
The general procedure for the synthesis of 5-bromo-4-fluoro-2-methylaniline from 2-nitro-4-bromo-5-fluorotoluene was as follows: a mixture of iron powder (17.8 g, 318 mmol) and ammonium chloride (5.10 g, 95.4 mmol) in water (100 mL) was heated to reflux for 30 minutes. Subsequently, 4-bromo-3-fluoro-6-nitrotoluene (18.6 g, 79.5 mmol) was slowly added to this hot mixture and the reaction mixture was continued to reflux for 48 hours. Upon completion of the reaction, the mixture was cooled to room temperature and extracted with ethyl acetate (3 x 200 mL). The organic phases were combined, washed sequentially with water (3 × 300 mL) and brine (300 mL), dried over anhydrous sodium sulfate and concentrated. The residue was purified by fast column chromatography (silica gel as stationary phase and hexane solution of 20% ethyl acetate as eluent) to afford 11.7 g of 5-bromo-4-fluoro-2-methylaniline as a light yellow solid in 72% yield. The product was characterized by 1H NMR (CDCl3, 300 MHz): δ 6.82 (m, 2H), 3.49 (bs, 2H), 2.11 (s, 3H).
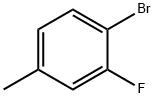
452-74-4
306 suppliers
$17.00/25g

627871-16-3
141 suppliers
$6.00/250mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1: 92 percent / HNO3; H2SO4 / 0 °C
2: 63 percent / SnCl2 / ethyl acetate
References:
Gopalsamy, Ariamala;Aplasca, Alexis;Ciszewski, Gregory;Park, Kaapjoo;Ellingboe, John W.;Orlowski, Mark;Feld, Boris;Howe, Anita Y.M. [Bioorganic and Medicinal Chemistry Letters,2006,vol. 16,# 2,p. 457 - 460]

224185-19-7
201 suppliers
$7.00/1g

627871-16-3
141 suppliers
$6.00/250mg