
5-BROMO-3-METHOXY-2-NITROPYRIDINE synthesis
- Product Name:5-BROMO-3-METHOXY-2-NITROPYRIDINE
- CAS Number:152684-26-9
- Molecular formula:C6H5BrN2O3
- Molecular Weight:233.02
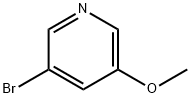
50720-12-2

152684-26-9
General procedure for the synthesis of 5-bromo-3-methoxy-2-nitropyridine from 3-bromo-5-methoxypyridine: 3-bromo-5-methoxypyridine (20.91 g, 0.11 mol) was dissolved in concentrated sulphuric acid (63 ml), and nitric acid (5.2 ml, 0.12 mol) was slowly added dropwise in an ice-bath under cooled conditions, and the reaction mixture was stirred for 20 h at room temperature. After completion of the reaction, the mixture was carefully poured into ice water with continuous stirring. The precipitated solid was collected by filtration and washed with cold water. The resulting solid was dissolved in ethyl acetate and the organic layer was washed sequentially with saturated aqueous sodium bicarbonate and saturated brine, followed by drying with anhydrous magnesium sulfate. After filtration to remove the drying agent, the filtrate was concentrated under reduced pressure to afford the target product 5-bromo-3-methoxy-2-nitropyridine (21.97 g, 85% yield) in the form of a light yellow solid. The structure of the product was confirmed by 1H NMR (CDCl3, 400 MHz): δ 4.01 (3H, s), 7.68 (1H, d, J = 1.6 Hz), 8.16 (1H, d, J = 1.9 Hz).

50720-12-2
357 suppliers
$11.00/5g

152684-26-9
93 suppliers
$30.00/250mg
Yield: 85%
Reaction Conditions:
with sulfuric acid;nitric acid in water at 0; for 20 h;
Steps:
65.2
The compound (20.91 g, 0.11 mol) obtained in Step 1 was dissolved in concentrated sulfuric acid (63 ml), nitric acid (5.2 ml, 0.12 mol) was added under ice-cooling, and the mixture was stirred for 20 hr. The reaction mixture was gently poured into ice water and the mixture was stirred. The precipitated solid was filtered and washed with water. The obtained solid was dissolved in ethyl acetate, and the solution was washed with saturated aqueous sodium hydrogencarbonate solution and saturated brine, and dried over magnesium sulfate. After filtration, the filtrate was concentrated under reduced pressure to give the object product as a pale-yellow solid (21.97 g, yield 85%). 1H NMR(CDCl3 400 MHz) (δ) ppm: 4.01 (3H, s), 7.68 (1H, d, J=1.6 Hz), 8.16 (1H, d, J=1.9 Hz)
References:
Satoh, Motohide;Aramaki, Hisateru;Nakamura, Hiroshi;Inoue, Masafumi;Kawakami, Hiroshi;Shinkai, Hisashi;Matsuzaki, Yuji;Yamataka, Kazunobu US2006/84665, 2006, A1 Location in patent:Page/Page column 70
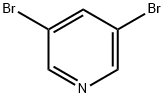
625-92-3
500 suppliers
$6.00/10g

152684-26-9
93 suppliers
$30.00/250mg