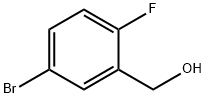
5-Bromo-2-fluorobenzylamine hydrochloride synthesis
- Product Name:5-Bromo-2-fluorobenzylamine hydrochloride
- CAS Number:99725-13-0
- Molecular formula:C7H6BrFO
- Molecular Weight:205.02
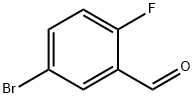
93777-26-5

99725-13-0
The general procedure for the synthesis of 5-bromo-2-fluorobenzyl alcohol from 5-bromo-2-fluorobenzaldehyde was as follows: sodium borohydride (8.39 g, 222 mmol) was slowly added to an ethanol (EtOH) solution of 5-bromo-2-fluorobenzaldehyde (30.0 g, 148 mmol) at 0 °C. The reaction mixture was stirred at 0 °C, followed by a gradual warming to room temperature over 15 h. The reaction was carried out at 0 °C. The reaction was carried out at 0 °C. The reaction was carried out at 0 °C. Upon completion of the reaction, the mixture was again cooled to 0 °C and the reaction was quenched with saturated aqueous ammonium chloride (NH4Cl) solution. The reaction mixture was extracted with ethyl acetate (EtOAc) and the organic layer was separated. The organic layer was subsequently washed with brine, dried over anhydrous magnesium sulfate (MgSO4), filtered and concentrated under reduced pressure to afford 5-bromo-2-fluorobenzyl alcohol (30.3 g, quantitative yield) as a white solid, which can be used in subsequent reactions without further purification. [M-OH]+ 187.

93777-26-5
369 suppliers
$5.00/5g

99725-13-0
127 suppliers
$7.00/250mg
Yield: 100%
Reaction Conditions:
Stage #1:5-bromo-2-fluorobenzaldehyde with sodium tetrahydroborate;ethanol at 0 - 20; for 15 h;Inert atmosphere;
Stage #2: with water;ammonium chloride in ethanol at 0;
Steps:
4
Preparation Example 4(5-Bromo-2-fluorophenyl)methanolTo a solution of 5-bromo-2-fluorobenzaldehyde (30.0 g, 148 mmol) in EtOH was added sodium borohydride (8.39 g, 222 mmol) at 0 °C. The resulting mixture was stirred with gradual warming to ambient temperature over 15 h, re-cooled to 0 °C, and quenched with aq. saturated NH4C1 solution. The mixture was extracted with EtOAc. The organic layer was washed with brine, dried over MgS04, filtered and evaporated in vacuo to yield the titled compound (30.3 g, quantitative) as a white sold, which was carried on to the next step without further purification.[M-OH"]+ 187.
References:
GREEN CROSS CORPORATION;LEE, Jinhwa;KIM, Jeongmin;LEE, Suk Ho;LEE, Junwon;SONG, Kwang-Seop;PARK, Eun-Jung;KIM, Min Ju;SEO, Hee Jeong;LEE, Sung-Han;SON, Eun Jung;KIM, Jong Yup;KANG, Suk Youn;KONG, Younggyu WO2011/159067, 2011, A2 Location in patent:Page/Page column 51

67-56-1
782 suppliers
$7.29/5ml-f

93777-26-5
369 suppliers
$5.00/5g

99725-13-0
127 suppliers
$7.00/250mg
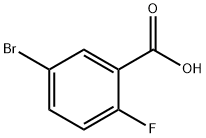
146328-85-0
290 suppliers
$6.00/1g

99725-13-0
127 suppliers
$7.00/250mg
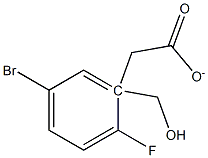
99748-26-2
1 suppliers
inquiry

99725-13-0
127 suppliers
$7.00/250mg

51437-00-4
275 suppliers
$6.00/5g

99725-13-0
127 suppliers
$7.00/250mg