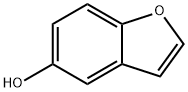
5-Hydroxybenzo[b]furan synthesis
- Product Name:5-Hydroxybenzo[b]furan
- CAS Number:13196-10-6
- Molecular formula:C8H6O2
- Molecular Weight:134.13
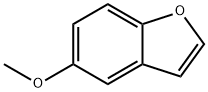
13391-28-1
![5-Hydroxybenzo[b]furan](/CAS/GIF/13196-10-6.gif)
13196-10-6
GENERAL STEPS: 5-methoxybenzofuran (0.5 g, 3.37 mmol) was dissolved in dichloromethane (DCM, 7 mL) under ice-bath cooling conditions and boron tribromide (3.4 mL, 3.37 mmol, 1 M DCM solution) was added slowly. The reaction mixture was stirred at 0 °C for 1 h. An equal amount of boron tribromide (3.4 mL) was added again. Subsequently, the reaction mixture was brought to room temperature and stirring was continued for 2 hours. The progress of the reaction was monitored by thin layer chromatography (TLC) and after confirming the completion of the reaction, the reaction mixture was slowly poured into ice water and the pH was adjusted with sodium carbonate (Na2CO3) to 7. The aqueous phase was extracted with dichloromethane (DCM, 2×), the organic phases were combined and washed with saturated brine, dried over anhydrous sodium sulphate (Na2SO4) and concentrated under reduced pressure. The resulting light brown solid product (0.36 g, yield 79.6%) was pure enough to meet the requirements of the subsequent reaction and did not require further purification. The structure of the product was confirmed by 1H NMR (400 MHz, CD3OD): δ 7.59 (d, J = 2.0 Hz, 1H), 7.35 (d, J = 9.2 Hz, 1H), 7.01 (d, J = 2.4 Hz, 1H), 6.82 (dd, J = 8.8 Hz, J = 2.8 Hz, 1H), 6.67 (m, 1H), 4.73 (s 1H).

150-76-5
807 suppliers
$5.00/5 g

2032-35-1
490 suppliers
$5.00/10g
![5-Hydroxybenzo[b]furan](/CAS/GIF/13196-10-6.gif)
13196-10-6
117 suppliers
$88.00/100mg
Yield:-
Reaction Conditions:
Stage #1:4-methoxy-phenol;Bromoacetaldehyde diethyl acetal with potassium hydroxide
Stage #2:
Steps:
Compound 9: 5-Methoxybenzofuran
4-Methoxyphenolwas alkylated with 2-bromoacetaldehyde diethyl acetal in thepresence of KOH. The obtained ether was subsequently cyclizedwith PPA, affording 5-hydroxybenzofuran. Compound9 was obtained by methylation of alcohol described as compound4. Compound 9 was obtained by methylation of alcoholdescribed as compound 4. Colorless oil, 1H-NMR (500 MHz,CDCl3) δ: 3.85 (3H, s), 6.71 (1H, d, J=2.3 Hz), 6.92 (1H, dd,J=2.3, 9.2 Hz), 7.06 (1H, d, J=2.3 Hz), 7.41 (1H, d, J=9.2 Hz),7.60 (1H, d, J=2.3 Hz). 13C-NMR (125 MHz, CDCl3) δ: 56.0,103.6, 106.8, 111.9, 113.2, 128.1, 145.8, 150.0, 156.0. Its spectraldata are in accordance with previously reported data.
References:
Yamaguchi, Yuki;Akimoto, Ichie;Motegi, Kyoko;Yoshimura, Teruki;Wada, Keiji;Nishizono, Naozumi;Oda, Kazuaki [Chemical and Pharmaceutical Bulletin,2013,vol. 61,# 10,p. 997 - 1001]

13391-28-1
130 suppliers
$31.00/100mg
![5-Hydroxybenzo[b]furan](/CAS/GIF/13196-10-6.gif)
13196-10-6
117 suppliers
$88.00/100mg

69034-13-5
35 suppliers
$35.70/5ml
![5-Hydroxybenzo[b]furan](/CAS/GIF/13196-10-6.gif)
13196-10-6
117 suppliers
$88.00/100mg

150-76-5
807 suppliers
$5.00/5 g
![5-Hydroxybenzo[b]furan](/CAS/GIF/13196-10-6.gif)
13196-10-6
117 suppliers
$88.00/100mg

331834-13-0
103 suppliers
$19.00/100mg
![5-Hydroxybenzo[b]furan](/CAS/GIF/13196-10-6.gif)
13196-10-6
117 suppliers
$88.00/100mg