
5-((4-Ethylpiperazin-1-yl)methyl)pyridin-2-amine synthesis
- Product Name:5-((4-Ethylpiperazin-1-yl)methyl)pyridin-2-amine
- CAS Number:1180132-17-5
- Molecular formula:C12H20N4
- Molecular Weight:220.31

121-44-8
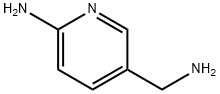
156973-09-0

1180132-17-5
To a 500 mL four-necked round-bottomed flask equipped with a thermometer and condenser tube were added 100 g of 1,2-dichloroethane, 12.5 g (0.1 mol) of 5-(aminomethyl)pyridin-2-amine (IV) and 30.0 g of potassium carbonate. A solution consisting of 25.0 g (0.15 mol) N,N-diethylethylamine and 20 g of 1,2-dichloroethane was added slowly and dropwise over a period of about 2 hours at 30 to 35 °C. The reaction temperature was maintained at 30~35°C and stirring was continued for 6 hours. After completion of the reaction, it was cooled to 20~25°C and layered. The aqueous layer was extracted twice with 1,2-dichloroethane at 20 g. The organic phases were combined and the 1,2-dichloroethane was removed by distillation. To the residue was added 75 g of methyl tert-butyl ether and 0.5 g of activated charcoal and stirred at 80~82°C for 1 hr. After hot filtration, the filtrate was cooled to 0~5°C for crystallization, filtered and dried to afford 18.2 g of 5-((4-ethylpiperazin-1-yl)methyl)pyridin-2-amine as a white solid in 82.7% yield and 99.3% purity of the liquid phase.
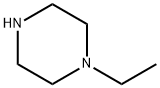
5308-25-8
466 suppliers
$15.00/5G
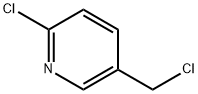
70258-18-3
528 suppliers
$5.00/5g

1180132-17-5
222 suppliers
$25.00/250mg
Yield:1180132-17-5 94%
Reaction Conditions:
at 60 - 70; for 2 h;Green chemistry;Time;
Steps:
1 Example 1 Synthesis of 1-(6-chloro-pyridin-3-ylmethyl)-4-ethyl-piperazine (II)
2-Chloro-5-chloromethylpyridine (162 g, 1.0 mol), tap water 300 mL, and N-ethylpiperazine (171 g, 1.5 mol) were added to a 1 L reaction flask.Raise to 60-70 ° C, stir for 2 h, cool to room temperature,Potassium carbonate (138 g, 1.0 mol) was added and stirred for 10 minutes, the aqueous phase was separated, and dried to give 225 g of product, yield 94%.
References:
CN108440401,2018,A Location in patent:Paragraph 0014; 0032-0040; 0047; 0048

5308-25-8
466 suppliers
$15.00/5G
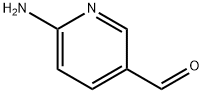
69879-22-7
139 suppliers
$70.00/250mg

1180132-17-5
222 suppliers
$25.00/250mg

121-44-8
897 suppliers
$9.00/5g

156973-09-0
60 suppliers
$199.00/500mg

1180132-17-5
222 suppliers
$25.00/250mg
![Piperazine, 1-[(6-broMo-3-pyridinyl)Methyl]-4-ethyl-](/CAS/20150408/GIF/1231930-25-8.gif)
1231930-25-8
72 suppliers
inquiry

1180132-17-5
222 suppliers
$25.00/250mg

5308-25-8
466 suppliers
$15.00/5G

1180132-17-5
222 suppliers
$25.00/250mg