
TERT-BUTYL 5-HYDROXY-3,4-DIHYDROQUINOLINE-1(2H)-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 5-HYDROXY-3,4-DIHYDROQUINOLINE-1(2H)-CARBOXYLATE
- CAS Number:497068-73-2
- Molecular formula:C14H19NO3
- Molecular Weight:249.31

24424-99-5
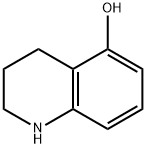
61468-43-7

497068-73-2
GENERAL STEPS: Example 9 Synthesis of (2-chloro-3-trifluoromethyl-benzyl)-(2,2-diphenyl-ethyl)-[3-(1-methyl-1,2,3,4-tetrahydro-quinolin-5-yloxy)-propyl]-amine. a) Preparation of tert-butyl 5-hydroxy-3,4-dihydro-2H-quinoline-1-carboxylate: to a stirred 5-hydroxytetrahydroquinoline ( 0.86 g, 5.34 mmol) to a solution of dioxane (25 mL) was added di-tert-butyl dicarbonate (1.16 g, 5.34 mmol). The reaction mixture was stirred at room temperature for 24 hours and then concentrated under vacuum to give an oily product. The crude product was diluted with ether and washed with water. The organic layer was separated, dried with anhydrous magnesium sulfate, filtered and concentrated to give a thick oily product. Recrystallization by dichloromethane-hexane mixed solvent resulted in 1.25 g of yellow crystal product in 94% yield.

24424-99-5
867 suppliers
$13.50/25G

61468-43-7
41 suppliers
$198.00/250mg

497068-73-2
19 suppliers
$76.00/100mg
Yield:497068-73-2 94%
Reaction Conditions:
in 1,4-dioxane; for 24 h;
Steps:
9.a
Example 9 (2-CHLORO-3-TRIFLUOROMETHYL-BENZYL)- (2, 2-DIPHENYL-ETHYL)- [3- (1-METHYL-1, 2,3, 4- tetrahydro-quinolin-5-yloxy)-propyl]-amine a) 5-Hydroxy-3, 4-DIHYDRO-2H-QUINOLINE-1-CARBOXYLIC acid tert-butyl ester To a stirring solution of 5-hydroxy tetrahydroquinoline (C. J. Cavallito, T. H. Haskell, J. Am. Chem. Soc. 1944,66, 1166) (0.86 g, 5.34 MMOL) in dioxane (25 ML) under argon was added DI-TEFF-BUTYIDICARBONATE (1.16 g, 5.34 MMOL). The reaction mixture was stirred for 24 h and concentrated in vacuo to afford an oil. The crude carbamate was diluted with diethyl ether, and water was added. The ether layer was separated, dried over MGS04, filtered, and concentrated to afford a thick oil. Recrystallization from methylene chloride-hexane afforded yellow crystals, 1.25 g (94%).
References:
WO2005/23188,2005,A2 Location in patent:Page/Page column 34

6931-19-7
77 suppliers
$24.00/1g

497068-73-2
19 suppliers
$76.00/100mg
30389-37-8
17 suppliers
inquiry

497068-73-2
19 suppliers
$76.00/100mg