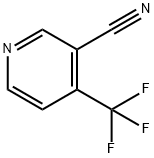
4-(Trifluoromethyl)nicotinonitrile synthesis
- Product Name:4-(Trifluoromethyl)nicotinonitrile
- CAS Number:13600-43-6
- Molecular formula:C7H3F3N2
- Molecular Weight:172.11
![2-Propenenitrile, 3-[(4,4,4-trifluoro-3-oxo-1-buten-1-yl)amino]-, (2Z)-](/CAS/20210305/GIF/533932-97-7.gif)
533932-97-7
![2-Propenenitrile, 3-[(4,4,4-trifluoro-3-oxo-1-buten-1-yl)amino]-, (2E)-](/CAS/20210305/GIF/533932-96-6.gif)
533932-96-6

13600-43-6
The general procedure for the synthesis of 3-cyano-4-trifluoromethylpyridine (Compound VIIb) from Compound (CAS:533932-97-7) and Compound (CAS:533932-96-6) is as follows (refer to Example 5): Step F: To a solution of 28% sodium methanol (580 mg, 3.0 mmol) in methanol (5 mL) was added a methanol solution of 3-[(4,4,4-trifluoro-3-oxo-1-butenyl)amino]-2-propenenenitrile (a mixture of IIa and IIb; 380 mg, 2.0 mmol), and the reaction mixture was stirred and then refluxed for 2 hours at room temperature. After completion of the reaction, the reaction solution was poured into water and extracted with ethyl acetate. The organic layers were combined, washed with brine, dried over magnesium sulfate and then concentrated. The resulting residue was purified by silica gel column chromatography (elution solvent: hexane/ethyl acetate = 3/1) to give 195 mg (56.5% yield) of the target compound 3-cyano-4-trifluoromethylpyridine. 1H-NMR spectrum (200 MHz, CD3OD) δ (ppm): 9.11 (1H, s), 9.03 (1H, d, J=5.1 Hz), 7.72 (1H, d, J=5.1 Hz).
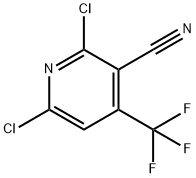
13600-42-5
165 suppliers
$9.00/250mg

13600-43-6
180 suppliers
$32.00/1g
Yield: 98.5%
Reaction Conditions:
with pyridine;hydrogen in ethanol at 30; under 760.051 Torr; for 4 h;Green chemistry;Reagent/catalyst;Temperature;Solvent;
Steps:
1-8
Put 25g of 2,6-dichloro-3-cyano-4-trifluoromethylpyridine into a 250 mL four-necked flask, add 100g ethanol, 21g pyridine, and 0.75g homemade Ni-Fe/C bimetallic load in sequence Type catalyst, magnetic stirring, 30 °C normal pressure hydrogenation reaction for 4 hours, after sampling and testing qualified, use Buchner funnel to filter and recover the catalyst, heat to 95-100 to vaporize ethanol and pyridine, extract with toluene, desolvate to obtain 3 -Cyano-4-trifluoromethylpyridine, the content is 98.5%, and the yield is 98.5%.
References:
Shandong Jingbo Biological Technology Co., Ltd.;Zhu Guofu;Li Yanfang;Cheng Daoquan;Wang Lei;Xiang Chuan;Wang Guangjin;Qiu Jinxian;Gao Huan;Yu Lianyou;Wang Hui;Cheng Xiaotong CN112110855, 2020, A Location in patent:Paragraph 0006; 0024-0038
![2-Propenenitrile, 3-[(4,4,4-trifluoro-3-oxo-1-buten-1-yl)amino]-, (2Z)-](/CAS/20210305/GIF/533932-97-7.gif)
533932-97-7
0 suppliers
inquiry

13600-43-6
180 suppliers
$32.00/1g
![2-Propenenitrile, 3-[(4,4,4-trifluoro-3-oxo-1-buten-1-yl)amino]-, (2E)-](/CAS/20210305/GIF/533932-96-6.gif)
533932-96-6
0 suppliers
inquiry

13600-43-6
180 suppliers
$32.00/1g
100-54-9
573 suppliers
$5.00/10g

13600-43-6
180 suppliers
$32.00/1g
216431-85-5
209 suppliers
$8.00/1g