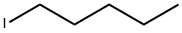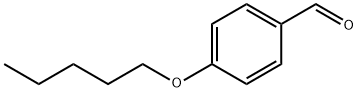
4-N-PENTYLOXYBENZALDEHYDE synthesis
- Product Name:4-N-PENTYLOXYBENZALDEHYDE
- CAS Number:5736-91-4
- Molecular formula:C12H16O2
- Molecular Weight:192.25
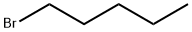
110-53-2

123-08-0

5736-91-4
The general procedure for the synthesis of 4-n-pentyloxybenzaldehyde from 1-bromopentane and p-hydroxybenzaldehyde is as follows: it was prepared by Williamson ether synthesis. This was done as follows: 4-hydroxybenzaldehyde (8.72 g, 71.5 mmol) was added to a solution of ethanol (60 mL) containing potassium hydroxide (4 g). Subsequently, n-alkyl halides (71.5 mmol) were added to the mixture (methyl iodide was used for the preparation of A1, while n-alkyl bromide was used for the preparation of A1-A13) and the reaction mixture was heated under reflux conditions. The reaction progress was monitored by thin layer chromatography (TLC) using chloroform as eluent to determine the optimal time for each reaction. After completion of the reaction, ethanol was removed by evaporation and the residue was dissolved in ether. The ether layer was washed sequentially with 10% aqueous potassium hydroxide and water until the pH of the wash solution reached 7. Finally, the organic layer was dried with anhydrous sodium sulfate, and evaporation afforded the target product, 4-n-pentyloxybenzaldehyde. The specific reaction conditions for each compound are detailed in Table 1.
Yield: 95.3%
Reaction Conditions:
with potassium carbonate in acetone at 60 - 70; for 24 h;Inert atmosphere;
Steps:
Preparation of 4-(pentyloxy)benzaldehyde (14)
To a suspension of 4-hydroxy benzaldehyde 13 (30.0 g, 0.245 mol) and powdered potassium carbonate (135.6 g, 0.982 mol) in acetone (600 mL, 20.0 Vol), 1-bromopentane (48.23 g, 0.319 mol) was slowly added at RT over the period of 20 min then heated to 60-70 °C and stirred for 24h. After completion of starting material, reaction mixture was cooled to RT, filtered through a celite bed and washed with acetone (200 mL).The filtrate was evaporated under reduced pressure to obtain crude product which was dissolved in ethylacetate (500 mL) and washed with water (1 x 100 mL) and brine (1 x 100 mL) and dried over anhy. Na2SO4 to afford crude which was passed through short silica gel column eluting with 10% EtOAc-Hexane to furnish 4-(pentyloxy)benzaldehyde 14 (45 g, 95.3% yield) as a colorless oil. 1H NMR (400 MHz, CDCl3) : δ 9.87 (s, 1H, H-1), 7.82 (d, J = 9.0 Hz, 2H, H-3 & H-3’), 6.99 (d, J = 8.5 Hz, 2H, H-4 & H-4’), 4.03 (t, J = 6.5 Hz, 2H, H-6), 1.82-1.73 (m, 2H, H-7), 1.49-1.37 (m, 4H, H-8 & H-9), 0.94 (t, J = 7.2 Hz, 3H, H-10); 13C NMR (100 MHz, CDCl3) δ 191.0 (C-1), 164.4 (C-5), 132.1 (C-3 & C-3’), 129.9 (C-2), 114.9 (C-4 & C-4’), 68.6 (C-6), 28.9 (C-7), 28.3 (C-8), 22.6 (C-9), 14.2 (C-10); Mass (ESI) m/z= 193.15 [M+H]+
References:
Rao, Pallavi;Hussain, Ismail;Rao, Venkataramanarao;Sen, Saikat;Oruganti, Srinivas [Synthetic Communications,2019,vol. 49,# 17,p. 2180 - 2187] Location in patent:supporting information

123-08-0
961 suppliers
$5.00/10g

5736-91-4
112 suppliers
$40.10/5g

543-59-9
195 suppliers
$20.00/25ml

123-08-0
961 suppliers
$5.00/10g

5736-91-4
112 suppliers
$40.10/5g

2050-04-6
16 suppliers
inquiry

5736-91-4
112 suppliers
$40.10/5g