
4-Morpholinopiperidine synthesis
- Product Name:4-Morpholinopiperidine
- CAS Number:53617-35-9
- Molecular formula:C9H18N2O
- Molecular Weight:170.25

415967-79-2

53617-35-9
General procedure for the synthesis of 4-(4-piperidinyl)morpholine from N-benzyl-4-(4-morpholinyl)piperidine: 41.59 g (0.16 mol) of N-benzyl-4-(4-morpholinyl)piperidine was dissolved in 400 mL of methanol, and 5.2 g of 10% palladium/carbon catalyst was added. The hydrogenation reaction was carried out at room temperature for 18 hours at 50 psi hydrogen pressure. Upon completion of the reaction, the catalyst was removed by filtration and the filtrate was concentrated. A colorless oily substance was obtained, which was left to crystallize. The yield was 25.29 g with 93% yield.

415967-79-2
2 suppliers
inquiry

53617-35-9
245 suppliers
$10.00/1g
Yield:53617-35-9 100%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in isopropyl alcohol at 80; under 3750.38 Torr; for 7 h;Autoclave;Reagent/catalyst;
Steps:
11 Example 11
The compound (3) (120 g; 0.46 mol), 2-propanol (276 g), and the palladium/carbon catalyst (0.3 g in terms of metallic palladium) were placed in an autoclave with a capacity of 1 L, and then the whole was stirred at 80° C., under a hydrogen pressure of 0.5 MPa for 7 hours. After cooling the reaction, the catalyst was filtered and washed with 2-propanol to obtain a filtrate. The resulting filtrate was analyzed by gas chromatography. As a result, the yield of the compound (4) analyzed by the internal standard method was 97%, and the area percentage (excluding the solvent and the compounds with low boiling points) of the compound (4) was 100% (Table 2).
References:
US2019/210968,2019,A1 Location in patent:Paragraph 0093

334942-09-5
2 suppliers
inquiry

53617-35-9
245 suppliers
$10.00/1g
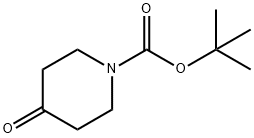
79099-07-3
0 suppliers
$5.00/5g

53617-35-9
245 suppliers
$10.00/1g
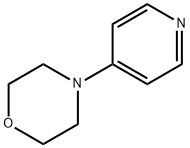
2767-91-1
121 suppliers
$12.00/1g

53617-35-9
245 suppliers
$10.00/1g
![Benzonitrile, 4-[4-(4-morpholinyl)-1-piperidinyl]-](/CAS/20210305/GIF/186650-77-1.gif)
186650-77-1
1 suppliers
inquiry

1194-02-1
530 suppliers
$6.00/10g

53617-35-9
245 suppliers
$10.00/1g