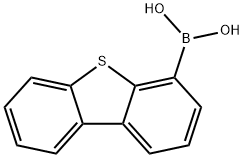
4-Dibenzothiopheneboronic acid synthesis
- Product Name:4-Dibenzothiopheneboronic acid
- CAS Number:108847-20-7
- Molecular formula:C12H9BO2S
- Molecular Weight:228.07

97511-05-2
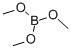
121-43-7

108847-20-7
The general procedure for the synthesis of dibenzothiophene-4-boronic acid from 4-bromodibenzothiophene and trimethyl borate was as follows: first, 4-bromodibenzothiophene was dissolved in anhydrous tetrahydrofuran (THF), and the reaction system was cooled to -78 °C. Under nitrogen protection, n-butyllithium (2.5 M hexane solution) was slowly added dropwise, maintaining the reaction temperature at -78 °C. After the dropwise addition, the reaction system was warmed to 0 °C and stirring was continued for 1 hour. Subsequently, the reaction system was again cooled to -78 °C and trimethyl borate was slowly added dropwise. After completion of dropwise addition, the reaction system was gradually warmed to room temperature and stirred for 12 hours. After completion of the reaction, the reaction mixture was slowly poured into 2N aqueous hydrochloric acid solution and stirred for 30 minutes. The aqueous phase was extracted with ether and the organic phase was combined. The organic phase was dried with anhydrous magnesium sulfate and filtered to remove the desiccant. The filtrate was concentrated under reduced pressure to give the crude product. The crude product was purified by column chromatography to afford the target product dibenzothiophene-4-boronic acid in 82% yield.

132-65-0
388 suppliers
$6.00/5g
6990-27-8
10 suppliers
$281.00/10ml

7732-18-5
498 suppliers
$13.50/100ML

108847-20-7
349 suppliers
$11.19/1G
Yield:108847-20-7 91%
Reaction Conditions:
Stage #1: dibenzothiophenewith N,N,N,N,-tetramethylethylenediamine;n-hexyllithium in hexane; for 2 h;Inert atmosphere;Reflux;
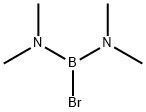
Stage #2: bromobis(dimethylamino)borane in hexane at 0;Inert atmosphere;
Stage #3: waterwith hydrogenchloride at 0 - 10; pH=2 - 3;
Steps:
5 Example 5
Under nitrogen protection, Dibenzothiophene (18.4 g, 0.1 mol) and n-hexane 85 mL were added to the reaction flask. Then TMEDA (12.8g, 0.11mol) was added, Become a clear solution with stirring, Then 2.3 M n-HexylLi hexane solution (48 mL, 0.11 mol) was added dropwise. Then the temperature was raised to reflux for 2 hours. Then cool down to 0 °C, Start adding BrB(NMe2)2 (18.7g, 99% purity, 0.105mol), After the addition is completed, Stirring reaction, TLC detection of raw materials disappeared, Filter out the solids produced, Cool down to 0 °C again, Quenched by adding 10% aqueous hydrochloric acid dropwise. Adjust pH=2-3, control temperature does not exceed 10 °C during quenching, Extracted with 180 mL of ethyl acetate twice. After the organic layer was evaporated to dryness, methanol (25 mL) was added and stirred until the crude product was completely dissolved. Then, about 4 times of methanol water (105 mL) was slowly added dropwise until all the solids were precipitated. After filtration and drying, 20.7 g of a white solid dibenzothiophene-4-boronic acid was obtained in a yield of 91%. HPLC: 99.1%, 1H NMR structure consistent, consistent with the literature.
References:
CN109456350,2019,A Location in patent:Paragraph 0019

97511-05-2
214 suppliers
$5.00/250mg
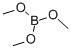
121-43-7
385 suppliers
$14.00/25mL

108847-20-7
349 suppliers
$11.19/1G

132-65-0
388 suppliers
$6.00/5g

5419-55-6
392 suppliers
$12.00/5g

108847-20-7
349 suppliers
$11.19/1G

97511-05-2
214 suppliers
$5.00/250mg

5419-55-6
392 suppliers
$12.00/5g

108847-20-7
349 suppliers
$11.19/1G

132-65-0
388 suppliers
$6.00/5g
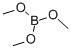
121-43-7
385 suppliers
$14.00/25mL

108847-20-7
349 suppliers
$11.19/1G