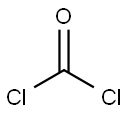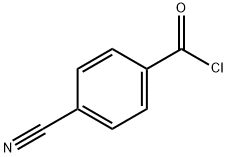
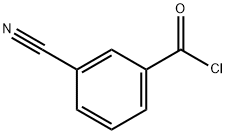
4-Cyanobenzoyl chloride synthesis
- Product Name:4-Cyanobenzoyl chloride
- CAS Number:6068-72-0
- Molecular formula:C8H4ClNO
- Molecular Weight:165.58
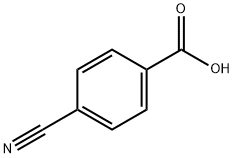
619-65-8

6068-72-0
4-Cyanobenzoic acid (30.00 g, 203.90 mmol) was added to thionyl chloride (29.11 g, 244.68 mmol) in batches under magnetic stirring. Subsequently, three drops of N,N-dimethylformamide (DMF) were added to the reaction system as a catalyst. The reaction mixture was heated to reflux with continuous stirring for 1.5 h. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the solution was distilled at 235 °C to afford the white solid product p-cyanobenzoyl chloride in 98% yield (5.50 g) with a melting point of 67.2-68.2 °C. The structure of the product was confirmed by the following characterization data: 1H NMR (600 MHz, DMSO-d6) δ 8.06 (d, J = 7.8 Hz, 2H), 7.96 (d, J = 7.8 Hz, 2H); 13C NMR (151 MHz, DMSO-d6) δ 165.9, 134.7, 132.6, 129.9, 118.1, 115.1; IR (KBr, νmax/cm-1)3101, 3051, 2229, 1772, 1739, 1597, 1401, 1287, 1211, 1167, 896, 851, 634; high-resolution mass spectrometry (HR-MS, ESI) m/z: [M + H]+ calculated value C8H4ClNO + H+ 166.0053, measured value 166.0054.

619-65-8
372 suppliers
$9.00/1g

6068-72-0
241 suppliers
$17.67/250mgs:
Yield:6068-72-0 98%
Steps:
62 Example 62
After dry nitrogen gas was introduced to the reaction mixture for one hour, acetonitrile was removed under ambient pressure, and was further removed under reduced pressure through distillation, to thereby obtain 16.2 g of p-cyanobenzoyl chloride (yield 98%, based on p-cyanobenzoic acid). The p-cyanobenzoyl chloride obtained had a purity of 99%.
References:
Showa Denko Kabushiki Kaisha US6433211, 2002, B1
1877-72-1
289 suppliers
$7.00/5g

6068-72-0
241 suppliers
$17.67/250mgs:
1711-11-1
112 suppliers
$17.00/250mg

3058-39-7
282 suppliers
$10.00/1g

122-04-3
36 suppliers
$15.00/1g

636-98-6
34 suppliers
$17.00/5g

6068-72-0
241 suppliers
$17.67/250mgs:
105-07-7
401 suppliers
$5.00/5g

6068-72-0
241 suppliers
$17.67/250mgs: