
4-CYANO-2-METHOXYPYRIDINE synthesis
- Product Name:4-CYANO-2-METHOXYPYRIDINE
- CAS Number:72716-86-0
- Molecular formula:C7H6N2O
- Molecular Weight:134.14

33252-30-1
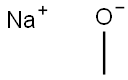
124-41-4

72716-86-0
General procedure for the synthesis of 4-cyano-2-methoxypyridine from 2-chloro-4-cyanopyridine and sodium methanol: To a solution of 2-chloro-4-cyanopyridine (1.38 g, 10 mmol) in dioxane (10 mL) was added a methanolic solution of sodium methanol (25%, 2.27 g, 10.5 mmol). The reaction mixture was heated to reflux for 5 h, followed by cooling to room temperature and standing overnight in a refrigerator. The precipitate was collected by filtration and washed with methanol. The filtrate was concentrated to about 10 mL and water (100 mL) was added. The precipitate was collected by filtration again and washed with water to give 4-cyano-2-methoxypyridine (1.93 g, 72% yield) as a white solid. Mass spectrum (electrospray positive ion mode) m/z 135 [M + H]+.

33252-30-1
397 suppliers
$8.00/5g

72716-86-0
94 suppliers
inquiry
Yield: 51%
Reaction Conditions:
with sodium in methanol;methanoldioxan;water
Steps:
3.a EXAMPLE 3
(a) Sodium (20.8 g) was dissolved in methanol (285 ml), a solution of 2-chloro-4-cyanopyridine (115.53 g) in methanoldioxan (1:1, 850 ml) was added and the mixture was boiled under reflux for 21/2 hours and was allowed to cool. The mixture was filtered and the volume of the filtrate was reduced by evaporation in 200 ml and water (400 ml) was added. The solid which precipitated out was filtered off to give 2-methoxy-4-cyanopyridine (57.2 g, 51%), m.p. 93-95.5°.
References:
Smith Kline & French Laboratories Limited US4255428, 1981, A

67-56-1
781 suppliers
$7.29/5ml-f

33252-30-1
397 suppliers
$8.00/5g
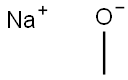
124-41-4
727 suppliers
$12.00/25g

72716-86-0
94 suppliers
inquiry

33252-30-1
397 suppliers
$8.00/5g

865-34-9
281 suppliers
$35.00/100g

72716-86-0
94 suppliers
inquiry