4-CHLORO-PYRIDINE-2-CARBONITRILE synthesis
- Product Name:4-CHLORO-PYRIDINE-2-CARBONITRILE
- CAS Number:19235-89-3
- Molecular formula:C6H3ClN2
- Molecular Weight:138.55
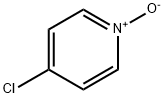
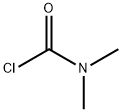
1121-76-2

7677-24-9

19235-89-3
4-Chloropyridine N-oxide (7.53 g, 58.1 mmol) and N,N-dimethylcarbamoyl chloride (9.36 g, 87.0 mmol) were mixed in acetonitrile (200 mL). Subsequently, trimethylcyanosilane (11.5 g, 116 mmol) was added slowly and dropwise to the reaction system. The reaction mixture was stirred at room temperature for 18 hours. Upon completion of the reaction, the mixture was combined with ethyl acetate and water for extraction. The organic layer was washed sequentially with saturated aqueous sodium bicarbonate and saturated saline, and then dried with magnesium sulfate. The solvent was removed by distillation and the residue obtained was purified by column chromatography (silica gel 200 g) using hexane-ethyl acetate (3:1, v/v) as eluent. The target fraction was collected and concentrated to give 4-chloro-2-cyanopyridine (8.05 g, 99% yield) as a light yellow oil. The product was characterized by 1H-NMR (CDCl3) and IR (KBr): 1H-NMR δ 7.54-7.56 (1H, m), 7.72 (1H, s), 8.63 (1H, d, J = 5.3 Hz); IR 2239, 1568, 1549, 1462, 1379, 1288, 1215, 844, 704 cm-1.

99586-65-9
241 suppliers
$14.00/5g

19235-89-3
264 suppliers
$10.00/1g
Yield:19235-89-3 90%
Reaction Conditions:
with triethylamine;trifluoroacetic anhydride in ethyl acetate at -5 - 20; for 1.25 h;
Steps:
5.1.2. 4-Chloropicolinonitrile (3)
The compound 2 (7.0 g, 44.7 mmol) and triethylamine (13 ml, 93.7mmol) were dissolved in dry EtOAc (50 ml) and the stirred solution was cooled to -5 °C in an ice-salt bath. Trifluoroacetic anhydride (13 ml, 92.2 mmol) was added dropwise to the chilled mixture over 45 min. The ice-salt bath was then removed and the reaction was warmed to room temperature and then stirred for another 30 min. The completion of reaction was detected by TLC, 10% aqueous K2CO3 (100 ml) was added, and the reaction mixture was allowed to stir for further 20 min. The mixture was extracted with EtOAc (3 × 50 ml), the combined organic layers were washed with brine (2 × 50 ml), dried over anhydrous Na2SO4, and concentrated under reduced pressure to give compound 3 (5.6 g, 90% yield) as a white solid, mp 84-85 °C; 1H NMR (DMSO-d6, 400 MHz): δ 7.54-7.56 (dd, J = 5.0 and 2.0 Hz, 1H), 7.74 (m, 1H), 8.65 (d, J = 5.0 Hz, 1H); ESI-MS m/z: 139[M+H]+; Anal. Calcd for C6H3ClN2 (%): C 52.01, H 2.18, N 20.22; Found: C 51.94, H 2.14, N 20.28.
References:
Zhan, Wenhu;Li, Yanyang;Huang, Weiping;Zhao, Yanjin;Yao, Zhenglin;Yu, Shanyou;Yuan, Shoujun;Jiang, Falong;Yao, Shan;Li, Shuxin [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 14,p. 4323 - 4329] Location in patent:experimental part

1121-76-2
212 suppliers
$8.00/1g

7677-24-9
393 suppliers
$19.00/5g

19235-89-3
264 suppliers
$10.00/1g

1121-76-2
212 suppliers
$8.00/1g

77-78-1
326 suppliers
$19.69/1ml

19235-89-3
264 suppliers
$10.00/1g

1121-76-2
212 suppliers
$8.00/1g

151-50-8
0 suppliers
$36.39/100g

19235-89-3
264 suppliers
$10.00/1g