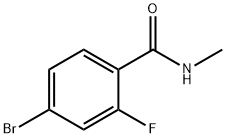
4-Bromo-2-fluoro-N-methylbenzamide synthesis
- Product Name:4-Bromo-2-fluoro-N-methylbenzamide
- CAS Number:749927-69-3
- Molecular formula:C8H7BrFNO
- Molecular Weight:232.05
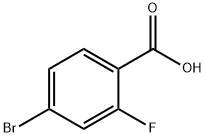
112704-79-7

74-89-5

749927-69-3
Step 1: Synthesis of 4-bromo-2-fluoro-N-methylbenzamide To a 100 mL round-bottomed flask were added 4-bromo-2-fluorobenzoic acid (3.0 g, 13.7 mmol), 2 M aqueous methylamine (34.3 mL, 68.5 mmol), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI, 6.6 g, 34.25 mmol), 1-hydroxybenzotriazole (HOBt, 2.8 g 20.6 mmol), N,N-diisopropylethylamine (DIPEA, appropriate amount) and N,N-dimethylformamide (DMF, 50 mL). The reaction mixture was stirred at room temperature for 16 hours. Upon completion of the reaction, the reaction was quenched by the addition of water (50 mL). The aqueous phase was separated and the organic phase was extracted with ethyl acetate (3 x 50 mL). The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography with the eluent ethyl acetate/petroleum ether (1:3, v/v) to afford the title compound 4-bromo-2-fluoro-N-methylbenzamide as a white solid (2.34 g, 74% yield). Mass spectrum (MS): [M+H]+ 232.

593-51-1
560 suppliers
$21.00/100g

112704-79-7
542 suppliers
$15.00/5 g

749927-69-3
301 suppliers
$40.00/1 g
Yield:749927-69-3 100%
Reaction Conditions:
with (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate;N-ethyl-N,N-diisopropylamine in dichloromethane at 20; for 2 h;
Steps:
Compound 49: (4-Bromo-2-fluoro-benzyl)-methyl-amine.
Compound 49: (4-Bromo-2-fluoro-benzyl)-methyl-amine. (1029) A mixture of 4-bromo-2-fluorobenzoic acid (1.0 equiv.), methylamine hydrochloride (1.1 equiv.), benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate (1.1 equiv.) and diisopropylethylamine (3.3 equiv.) in dichloromethane (0.20 mol.L-1) was stirred for 2 hours at room temperature. The reaction mixture was hydrolyzed and extracted twice with dichloromethane. The organic layers were combined, washed with brine, dried over MgSO4, concentrated, and purified by flash column chromatography on silica gel (10% to 50% EtOAc in cyclohexane) to afford the intermediate methylamide as a white solid in quantitative yield.
References:
MAVALON THERAPEUTICS LIMITED;SCHANN, Stephan;MAYER, Stanislas;MANTEAU, Baptiste WO2017/81483, 2017, A1 Location in patent:Page/Page column 228-229

112704-79-7
542 suppliers
$15.00/5 g

74-89-5
0 suppliers
$13.44/25ML

749927-69-3
301 suppliers
$40.00/1 g

74-89-5
0 suppliers
$13.44/25ML

749927-69-3
301 suppliers
$40.00/1 g
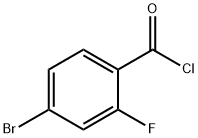
151982-51-3
157 suppliers
$9.00/250mg

74-89-5
0 suppliers
$13.44/25ML

749927-69-3
301 suppliers
$40.00/1 g

112704-79-7
542 suppliers
$15.00/5 g

749927-69-3
301 suppliers
$40.00/1 g