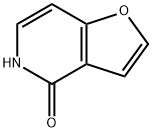
4,5-DIHYDRO-4-OXOFURO[3,2-C]PYRIDINE synthesis
- Product Name:4,5-DIHYDRO-4-OXOFURO[3,2-C]PYRIDINE
- CAS Number:26956-43-4
- Molecular formula:C7H5NO2
- Molecular Weight:135.12
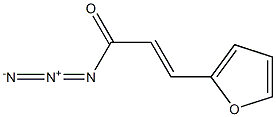
119924-26-4
![4,5-DIHYDRO-4-OXOFURO[3,2-C]PYRIDINE](/CAS/GIF/26956-43-4.gif)
26956-43-4
The general procedure for the synthesis of 4,5-dihydro-4-oxofuro[3,2]pyridine from (E)-3-(furan-2-yl)acryloyl azide was as follows: (E)-3-(furan-2-yl)acryloyl azide (150 g, 920 mmol) was added to toluene (800 mL) preheated to 100 °C and the reaction mixture was stirred for 30 min. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure. The residue was dissolved in o-dichlorobenzene (800 mL) and iodine (1 g) was added as a catalyst. The reaction mixture was stirred at 180 °C for 2 h, followed by evaporation under reduced pressure to remove the solvent. The residue was dissolved in methanol, the precipitate was collected by filtration and the filtrate was concentrated under reduced pressure. Finally, the residue was washed with diisopropyl ether to afford the target product 4,5-dihydro-4-oxofuro[3,2]pyridine (100 g, 80% yield). The product was characterized by 1H-NMR (DMSO-d6): δ 6.85 (1H, dd, J = 7.1, 1.0 Hz), 6.92 (1H, dd, J = 1.9, 1.0 Hz), 7.29 (1H, d, J = 7.1 Hz), 7.86 (1H, d, J = 1.9 Hz), 11.42 (1H, brs).

119924-26-4
13 suppliers
inquiry
![4,5-DIHYDRO-4-OXOFURO[3,2-C]PYRIDINE](/CAS/GIF/26956-43-4.gif)
26956-43-4
114 suppliers
$10.00/100mg
Yield:26956-43-4 80%
Reaction Conditions:
with iodine in 1,2-dichloro-benzene at 180; for 2 h;
Steps:
3
Reference Example 3 furo[3,2-c]pyridin-4(5H)-one; [Show Image] (2E)-3-(2-Furyl)acryloyl azide (150 g, 920 mmol) was added to toluene (800 mL) heated to 100°C, and the mixture was stirred for 30 min. The solvent was evaporated under reduced pressure. The residue was dissolved in orthodichlorobenzene (800 mL), and iodine (1 g) was added. The mixture was stirred at 180°C for 2 hr, and the solvent was evaporated under reduced pressure. The residue was dissolved in methanol, the precipitate was filtered off, and the filtrate was concentrated under reduced pressure. The residue was washed with diisopropyl ether to give the title compound (100 g, yield 80%). 1H-NMR (DMSO-d6) δ: (1H, dd, J =7.1, 1.0 Hz), 6.92 (1H, dd, J = 1.9, 1.0 Hz), 7.29 (1H, d, J= 7.1 Hz), 7.86 (1H, d, J = 1.9 Hz), 11.42 (1H, brs).
References:
Takeda Pharmaceutical Company Limited EP2100895, 2009, A1 Location in patent:Page/Page column 44
29080-01-1
0 suppliers
inquiry
![4,5-DIHYDRO-4-OXOFURO[3,2-C]PYRIDINE](/CAS/GIF/26956-43-4.gif)
26956-43-4
114 suppliers
$10.00/100mg

539-47-9
270 suppliers
$10.00/1g
![4,5-DIHYDRO-4-OXOFURO[3,2-C]PYRIDINE](/CAS/GIF/26956-43-4.gif)
26956-43-4
114 suppliers
$10.00/100mg

539-47-9
270 suppliers
$10.00/1g
![4,5-DIHYDRO-4-OXOFURO[3,2-C]PYRIDINE](/CAS/GIF/26956-43-4.gif)
26956-43-4
114 suppliers
$10.00/100mg

541-41-3
0 suppliers
$19.39/5g

539-47-9
270 suppliers
$10.00/1g
![4,5-DIHYDRO-4-OXOFURO[3,2-C]PYRIDINE](/CAS/GIF/26956-43-4.gif)
26956-43-4
114 suppliers
$10.00/100mg